As described in Chapter 2, surfactant/cosolvent flushing is an emerging technology, which at present, is generally in the technology development and demonstration phase. Consequently, a step-wise approach is needed to evaluate and potentially implement surfactant/cosolvent flushing as a remedial technology at a given site. Primary elements of the proposed step-wise approach include:
Through each of these steps, data are gathered and an ongoing analysis is performed to determine whether the prescribed remedial objective can be achieved and what the associated costs will be. In an effort to assist project managers, engineers, and scientists in implementing surfactant/cosolvent projects, this chapter describes each of the above steps in detail. Figure 5-1 presents a flow chart summarizing the sequenced approach and the re-evaluation after each stage.
In proceeding through these steps, it is important to remember that surfactant/cosolvent flushing is not a proven technology; therefore, "off the shelf" answers are not always available. Furthermore, because little information is available on any full-scale applications of surfactant/cosolvent flushing, the proposed step-wise approach presented here is an extrapolation based on small-scale field trials and experience with full-scale application of related technologies.
This section introduces a sequence of initial evaluations that lead to a basis for deciding if surfactant/cosolvent flushing is a promising approach for remediating a given site. The evaluations also result in an initial conceptualization of how surfactant/cosolvent flushing might be implemented at a site and what the associated costs may be. Comparison of these results to what might be achieved using other technologies provides a basis for deciding if surfactant/cosolvent flushing is a promising technology warranting further consideration. This section should be read by those interested in evaluating the applicability of surfactant/cosolvent flushing.
• Because surfactant/cosolvent flushing is primarily a NAPL remediation technology, the presence or absence of NAPL is a key screening consideration. This can be evaluated through development of a site conceptual model.
• In terms of remedial objectives, surfactant/cosolvent flushing by itself has the potential to remove substantial amounts of mass from a targeted interval, but likely will not restore water in the targeted interval to typical drinking water standards.
• Where pumping of free-product is feasible and/or stringent cleanup goals are targeted, surfactant/cosolvent flushing may be an element in a sequence of treatment technologies or "treatment train."
• Preliminary selection of a chemical system can be accomplished through consideration of contaminant type, hydrogeologic setting, and previous experience. This is an important step in developing a preliminary cost estimate.
• Fundamental to the overall feasibility of surfactant/cosolvent flushing is an ability to cycle fluids through a targeted interval in a reasonable period of time. Estimates of the time required to cycle a given number of pore volumes can be developed using screening calculations.
• Also fundamental to the feasibility of surfactant/cosolvent flushing are cost-effective methods to manage the produced fluids.
• Obtaining regulatory acceptance for delivery of chemical systems to subsurface targets can be a significant challenge due to concerns regarding potential adverse effects of the chemicals.
• To evaluate the applicability and approach of a surfactant/cosolvent flushing system, a preliminary conceptual design and cost estimate should be developed based on conceptual resolution of the above issues.
• As a final step, surfactant/cosolvent flushing should be compared to other technologies to determine if this is a cost-effective remedial alternative for a given site.
One of the more useful formats for site characterization data is a conceptual model. If a site conceptual model has not already been developed, it should be at this point. Conceptual models are discussed at length in Pankow and Cherry (1996), Cohen and Mercer (1993), and ASTM (1995). Conceptual models typically contain block diagrams that illustrate the spatial relationships of critical parameters. In assessing the feasibility of surfactant/cosolvent flushing, the following critical parameters should be included in a site conceptual model:
• Identification of NAPLs of concern, including their aqueous solubility, density, NAPL-water interfacial tension, and viscosity.
• The spatial distribution of target compound(s), including NAPL zones, aqueous dissolved plumes, and vapor plumes.
• The spatial distribution of sediment types, hydraulic conductivity, porosity, and secondary flow features such as root holes, joints, fractures, and sand seams.
• Conceptualizations of transport processes such as NAPL migration, redistribution to pools and residual, and diffusion into low-permeability regions.
Any remediation effort must begin with a clear idea of what needs to be accomplished. Most often, this is based on risk analysis and is documented in Risk Assessments, Feasibility Studies, Corrective Measures Studies, or Records of Decision. The remedial objectives must always be considered when evaluating and designing remediation alternatives.
In some situations, remedial objectives may not have been defined during the initial Feasibility Study. If this is the case, the range of possible remedial objectives must be considered when evaluating various technologies. Typical remedial objectives include:
Mass reduction can be achieved in many cases through surfactant/cosolvent flushing. Neither full restoration nor risk mitigation has been demonstrated using surfactant/cosolvent systems, although current research efforts may make these goals attainable in the future.
In conceptualizing a surfactant/cosolvent flushing application, it is important to recognize that it may be applied as a stand-alone technology or as an element in a more complex alternative or "treatment train." Surfactant/cosolvent flushing might be coupled with the technologies described below.
In many instances, it may be possible to remove "free-product" (that is, pooled NAPL that will flow to a well or drain ) from a target area prior to surfactant/cosolvent flushing. For example, "free-product" recovery was conducted as a preliminary step in seven of the 26 demonstrations described in Appendix A. Benefits include the following:
Various methods can be used to remove the mobile NAPL, including well skimming, groundwater depression with well skimming, vacuum-enhanced recovery (also called bioslurping), and waterflooding. Because waterflooding involves many of the same components as surfactant/cosolvent flushing, it is often an effective preliminary step.
Endpoint concentrations achieved in soil and groundwater after surfactant/cosolvent flushing may not meet cleanup goals. In this case, either natural or enhanced in-situ bioremediation may be needed to complete the remediation. Enhanced in-situ bioremediation could involve flushing water supplemented with oxygen through the subsurface. An example of this technique is described by Piontek and Simpkin (1994). For chlorinated solvents, the injection of organic substances such as acetate may enhance reductive dehalogenatives, thereby reducing the concentrations of chlorinated compounds remaining.
Natural or intrinsic bioremediation processes may be able to degrade or attenuate the contamination remaining after a surfactant/cosolvent flushing process. This is especially true for petroleum hydrocarbons.
As described in Chapter 4, a variety of physical processes can be exploited in the application of surfactants and/or cosolvents. At a general level, these processes may be categorized as NAPL solubilization and NAPL mobilization. Sabatini, et al. (1996) provide a good discussion of the relative merits of the two approaches. In general, mobilization removes a significantly larger mass of contaminants with a smaller amount of chemical. To date, projects addressing chlorinated solvents have focused largely on solubilization processes. The rationale is that the multiple order-of-magnitude shifts in interfacial tension associated with mobilization will lead to adverse downward migration of DNAPLs (Longino and Kueper, 1995). For the most part, in cases where the target is a low-density, low-solubility DNAPL (e.g., coal oil) or an LNAPL (e.g., JP-4), the focus has been on mobilization to achieve higher initial rates of mass removal. A combination of mobilization and solubilization also ma
y be employed. A "slug" of the NAPL could be removed using a mobilizing system and the remaining NAPL treated with a solubilizing system.
Once the preferred removal mechanism is selected, a range of possible surfactant/- cosolvent systems can be identified. The actual system to be used will be designed, tested, and evaluated in laboratory studies (as discussed in Section 5.3), but potential systems can be identified early on to allow preliminary cost estimates to be developed and to inform regulators of the nature of the chemicals to be injected into the subsurface. As discussed in Chapter 4, surfactant/cosolvent systems could include low surfactant concentration systems, high surfactant concentration systems, surfactant/alcohol systems, high alcohol concentration systems, low alcohol concentration systems, and alkaline-surfactant-polymer combination systems. Table 5-1 summarizes approaches that have been applied or are currently planned.
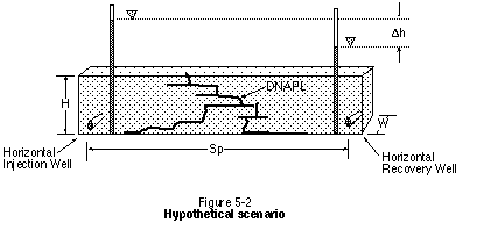
At a screening level, a fundamental concern is whether fluids can be cycled through the target in an acceptable period of time. Preliminary estimates of the time required to cycle a single pore volume of fluid through a target area can be developed using an idealized one-dimensional flow system, Darcy's equation, and best estimates of key parameters. As an example, consider the scenario illustrated in Figure 5-2. Ideally, the time to cycle one pore volume (tp) through the system is the volume of the pore space (Pv) divided by the rate of solution delivery/recovery (Qsol). This relationship is given as:
As discussed in Chapter 3, the time required to cycle a pore volume through the target zone can also be expressed in terms of a length and a groundwater velocity, as follows:
| Table 5-1
|
| Surfactant/Cosolvent Approaches Used
|
|
| Project
| Target Compound
| Removal Mechanism
| Chemical System
|
|---|
| Hill AFB, Utah-OU1 Cell #3
| Waste Jet Fuel and Light Lubricants Mixed with Chlorinated and Nonchlorinated Solvents
| Cosolvent Mobilization
| Cosolvents |
| Laramie, Wyoming-Large Field Demonstration
| Creosote-Based Wood Treating Oil-DNAPL (1.03 g/cm3, 54 centipoise)
| Mobilization and Solubilization with Mobility Control
| Surfactant, Alkaline Salts, Polymer
|
| Canadian Forces Base Borden, Ontario
| Tetrachloroethylene-DNAPL (1.56 g/cm3, 1.79 centipoise)
| Solubilization | Surfactant
|
| Fredricksburg, Virginia-DNAPL
| Creosite Based Wood Treating Oil with Mobility Control (1.03 g/cm3, 54 centipoise)
| Mobilization and Solubilization
| Alkaline Salts, Surfactant, and Polymer
|
| Hialeah County, Florida
| Hydraulic Oil (0.915 g/cm3, 130 centipoise)
| Mobilization with Mobility Control
| Alkaline Salts and Polymer
|
| Corpus Christi, Texas |
Carbon Tetrachloride-DNAPL (1.58 g/cm3, 0.965)
| Solubilization
| Surfactant
|
| Warren, Michigan
| Polychlorinated Biphenyls and "Oil"
| Mobilization and Solubilization
| Surfactant |
| Quebec City, Quebec |
"Cutting Oil" |
Not Specified
| Surfactant
|
| Hill AFB, Utah-Ethanol OU1
| Waste Jet Fuel and Light Lubricants Mixed with Chlorinated and Nonchlorinated Solvents
| Solubilization
| Solvent
|
| Hill AFB, Utah-Surfactant Solubilization, OU1, Cell #5
| Waste Jet Fuel and Light Lubricants Mixed with Chlorinated and Nonchlorinated Solvents
| Solubilization
| Surfactant
|
| Laramie, Wyoming-Small Field Demonstration
| Creosote-Based Wood Treating Oil-DNAPL (1.03 g/cm3, 54 centipoise)
| Mobilization and Solubilization with Mobility Control
| Surfactant, Alkaline Salts, Polymer
|
| Hill AFB, Utah-Cyclodextrin Solubilization, OU1, Cell #4
| Waste Jet Fuel and Light Lubricants Mixed with Chlorinated and Nonchlorinated Solvents
| Enhanced Solubility |
Sugar |
| Fort Worth, Texas | TCE DNAPL (Low ITF or Neutral Buoyancy)
| Tentatively Solubilization
| Tentatively SurfactantCosurfactant/ Polymer/Electrolyte
|
| Hill AFB, Utah-Surfactant with Cosolvent OU1, Cell #8
| Waste Jet Fuel and Light Lubricants Mixed with Chlorinated and Nonchlorinated Solvents
| Solubilization via Microemulsification
| Surfactant plus Cosolvent
|
| Hill AFB, Utah-OU2 Micellar Flood
| Chlorinated Solvents (TCE, TCA, PCE, and CTET) with Petroleum Hydrocarbons
| Solubilization with Low IFT Flood
| Surfactant, Cosurfactant, and Salinity
|
| Hill AFB, Utah-OU2 Foam Flood
| Chlorinated Solvents (TCE, TCA, PCE, and CTET) with Petroleum Hydrocarbons
| Solubilization with Mobility Control
| Surfactant Foam |
| Traverse City, Michigan
| PCE, TCE and Recalcitrant Jet Fuel
| Solubilization
| Surfactant
|
| L'Assomption, Quebec |
Waste Oil (DNAPL)
| Mobilization, Emulsification, and Solubilization
| Surfactant, Alcohol, Solvent
|
| Paducah, Kentucky
| TCE (DNAPL)
| Solubilization
| Surfactant
|
| Demont Station, Pennsylvania
| Polychlorinated Biphenyls
| Solubilization
| Surfactant/Alcohol Mixture
|
| Picatinny Arsenal, New Jersey
| TCE
| Enhanced Desorption
| Not Known at This Time
|
| Piketon, Ohio
| Mostly TCE (DNAPL) with Some PCBs and Other Chlorinated Solvents
| Solubilization
| Surfactant/ Cosurfactant/ Electrolyte
|
| Commercial Site, New Jersey
| Chlorinated Solvents and BTEX
| Not Known at This Time
| Not Known at This Time
|
| Hill AFB, Utah-Surfactant Mobilization, OU1 Cell #6
| Waste Jet Fuel and Light Lubricants Mixed with Chlorinate and Nonchlorinated Solvents
| Mobilization
| Surfactant Plus Cosolvent
|
|
Applying Darcy’s equation to describe Qsol, the time to deliver one pore volume can be described in terms of fundamental parameters, as follows:
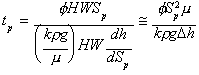 (4)
(4)
where H, W, and Sp are dimensions of the target zone as illustrated in Figure 5-2, f is the medium porosity, k is the average bulk permeability of the formation, g is the acceleration due to gravity, p is the density of the injected solution, and µ is the viscosity of the injected solution. The hydraulic gradient is given as dh/dSp, which is in turn represented by Dh/Sp, where Dh is the difference in hydraulic head between the injection and withdrawal drains. Equation (3) is a simplified approximation in that it assumes one-dimensional, steady-state flow under a uniform hydraulic gradient. The equation is useful, however, in examining the sensitivity to various system parameters. It should also be noted that the permeability term in Equation (3) could be modified by the bulk average relative permeability to provide a more accurate estimation of tp. For moderate to low
bulk retention capacities, however, the bulk average relative permeability to the water will be approximately equal to 1.
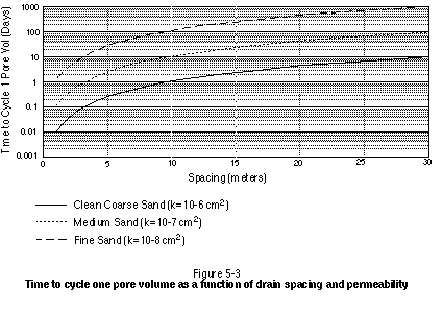
An application of Equation (3) using the parameters listed in Table 5-2 is presented in Figure 5-3. Figure 5-3 plots the time to cycle one pore volume as a function of spacing for three different target zone permeabilities. The permeability values correlate roughly to a well-sorted coarse sand, a medium sand, and a fine-grained sand. It is important to note that the times shown in Figure 5-3 are based on a highly simplified scenario. In actuality, non-uniform flow fields associated with spatially varying permeability and the delivery/ recovery system’s design will result in some portions of the target being flushed more quickly while other portions are flushed more slowly.
|
Table 5-2
|
|
Parameters for Equation (2)
|
|
|
Parameter
|
Definition
|
Assumed Value(s)
|
|
|
|
Porosity
|
0.25 |
|
Sp
|
Spacing
|
1 to 30 m |
|
k
|
Permeability
|
10-10,10-11, and 10-12 m2 |
|
g
|
Gravitational Constant
|
9.8 m/s |
|
|
Density of Solution
|
1000 kg/m3 |
|
Dh
|
Head Loss
|
3 m |
|
µ
|
viscosity
|
0.001 kg/m-s |
|
From Equation (3) and Figure 5-3, it can be seen that the time to cycle one pore volume between parallel horizontal delivery/recovery wells is a linear multiple of porosity and solution viscosity, and the square of the spacing. Furthermore, the time to cycle a single pore volume is inversely proportional to the permeability of the target zone and the change in head between the delivery and recovery trenches. The total time required to deliver a fixed number of pore volumes can be approximated by multiplying the required number of pore volumes by the time to flush one pore volume. Based on projects reviewed in Appendix A, the total number of pore volumes associated with chemical flooding can range between 4 and 15. Including pre- and post-waterflood, the historical number of pore volumes ranges between 6 and 120. Recognizing that surfactant/cosolvent flushing often requires multiple pore volumes, it is likely that between 1 and 10 years may be required to complete a full-scale surfa
ctant/cosolvent application at a moderate to large site.
Preferential Pathways
The actual time required to achieve a fixed amount of NAPL recovery will be strongly controlled by the presence of preferential pathways. Examples of major preferential pathways include:
• Slickenslides, root hollows, worm tubes, shell beds, and sand seams in coastal clays
• Glacial outwash gravel interbedded with tills
• Cinder beds and flow tops interbedded within basalt flows
• Permeable fracture systems within bedrock
• Fluvial channel deposits interbedded with finer point bar overbank deposits
It should also be noted that minor differences in permeability can have significant effects on the flow of fluids. For example, consider the simple layered system in Figure 5-4. Assuming that k1 is one order of magnitude less permeable than k2, it follows that it will take 10 times longer to flush a given number of pore volumes through k1. Order-of-magnitude variations in permeability are common, even in formations considered to be uniform.
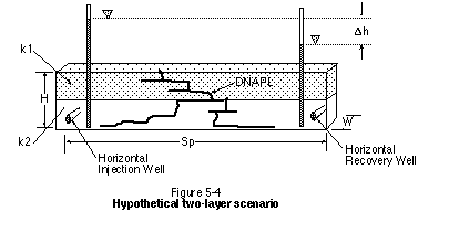
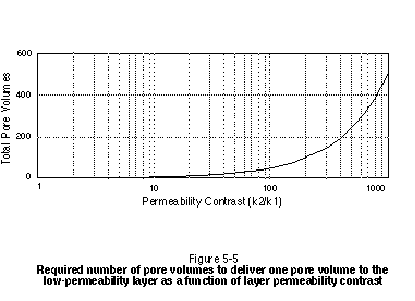
Another repercussion of the layered system is that a larger number of total system pore volumes will be required to deliver a single pore volume to the low-permeability layer. For example, assuming the two layers in Figure 5-4 are of equal thickness and porosity, a total of 5.5 system pore volumes will be required to deliver one pore volume to the low-permeability layer. Figure 5-5 illustrates the total number of pore volumes required to deliver one pore volume to the low-permeability layer for a ratio of k1 to k2 ranging from 1 to 1000.
Structural Traps
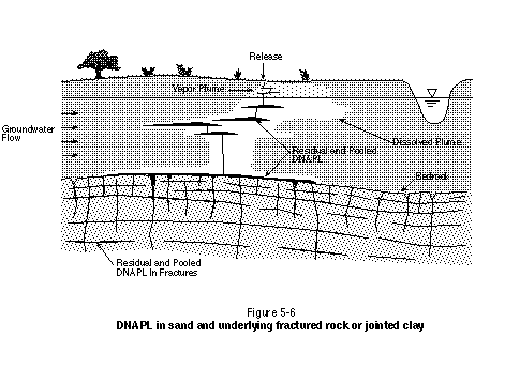
Both fractured rock and low-permeability soils (clays) form dual-porosity systems. Typically, this results in NAPL being distributed only in the secondary features (i.e., joints, root holes, burrows, etc.) and being precluded from the matrix by the high entry pressures associated with the low-permeability matrix. The primary implication of NAPL existing in discontinuous secondary features is that it may be difficult or impossible to contact with a chemical solution. Figure 5-6 illustrates a continuous porous medium (sand) overlying a dual-porosity system (fractured rock or jointed clay).
5.1.6 Conceptualization of Above-Ground Process Components
As described in Chapter 2, a successful surfactant/cosolvent technology also will require a number of above-ground process components. These components include systems for preparation of chemical flushing solutions, systems for delivery of the solutions to the subsurface, systems for recovery of the solutions, systems for treatment/reuse of the produced fluids, systems for residuals management, and systems for performance monitoring. These systems are discussed in more detail in Section 5.5. These components need to be conceptualized for a given chemical system and delivery/recovery approach at this stage in order to produce reasonable cost estimates and provide a complete picture of the remedial alternative being proposed.
5.1.7 Regulatory Acceptance
Historically, one of the primary limiting factors for surfactant/cosolvent applications has been obtaining permission from regulators to deliver chemicals to the subsurface. While the large number of current and planned surfactant/cosolvent projects indicates a growing acceptance for delivering chemicals to contaminated subsurface media, it is still a major issue at most sites. A critical step in screening the feasibility of surfactant/cosolvent flushing is to get a preliminary indication from involved regulatory agencies of potential constraints. Factors critical to obtaining regulatory acceptance include using "low impact" chemical solutions, designing recovery/delivery systems capable of capturing all delivered fluids, and site conditions that minimize unintentional contaminant spreading. Regulatory requirements are discussed in more detail in Section 5.5.4.
5.1.8 Preliminary Conceptual Design and Cost Estimate
The key issues raised in Sections 5.1.1 through 5.1.7 are summarized in Table 5-3. Once these issues are addressed, the next logical step is to develop a preliminary conceptual design and cost estimate for a surfactant/cosolvent flushing system. This can be accomplished following standard engineering practices. Examples of conceptual designs and cost estimates are presented in Chapter 6.
| Table 5-3 |
| Surfactant/Cosolvent Screening Issues
|
|
| Screening Issue |
Comment/Reference |
|---|
| Are NAPLs present? |
Based on historical land use and/or available site characterization, this is usually known.
Additional information on this topic is provided in Section 5.2.1. |
|
Are the soils sufficiently permeable to transmit a chemical system? |
Factors controlling flushing rates are introduced in Chapter 3. Methods for estimating flushing rates
are described in Section 5.1.5. Settings in which surfactant/cosolvent flushing field demonstrations have
been conducted or are planned are reviewed in Chapter 2. |
|
Do chemical systems exist that can remove the target compound at the site of concern?
|
Compounds that have been addressed to date are listed in Table 5-1. Factors to consider are introduced in Section 5.1.4. Rigorous attention is given to this topic in Section 5.3 (Laboratory Studies). |
| Once above ground, is there a cost-effective means to treat the produced fluids?
|
In many cases, it may be advantageous to separate the contaminants from the produced fluids and recycle or reuse a portion of the chemicals. This has the potential to significantly reduce the chemical and produced fluids treatment costs. Section 5.5.9 discusses options for produced fluids treatment, and Chapter 6 discusses costs. |
| Can a surfactant/cosolvent system achieve the remedial objectives?
|
Potential remedial objectives are described in Section 5.1.2. Chapter 2 discusses the contaminant removals that have been observed in field studies of surfactant/cosolvent demonstrations. Site-specific laboratory and field demonstrations will be needed to answer this issue. |
| From a regulatory perspective, is it feasible to deliver a chemical system to the target?
|
As introduced in Sections 5.1.7 and 5.5.4, consensus must be reached with regulatory groups as to the appropriateness of surfactant/cosolvent flushing. Further information on this topic may be found in USEPA (1996) and USEPA (1995). |
| Is surfactant/cosolvent flushing economically feasible?
|
Surfactant/cosolvent flushing may be a relatively expensive technology. Economic analyses should be conducted on both a net present worth and an annual cost basis when comparing technologies. Costs are discussed further in Chapter 6. |
| Within the constraints of current or planned land use, is it feasible to implement a surfactant/cosolvent remedy?
|
Fluid delivery, recovery, and treatment systems may involve an extensive network of piping and process equipment. Such systems may not be compatible with some land uses (e.g., process areas in petroleum refineries). |
| Are there any significant adverse risks associated with implementing Surfactant/cosolvent flushing at the site of interest?
|
Adverse vertical migration of DNAPLs, loss of target zone permeability, and production of difficult to manage residual fluids and/or solids are all potential issues to be addressed.
These issues are introduced in Chapter 4. |
5.1.9 Comparison to Other Remedial Alternatives
It is common in feasibility studies to compare and contrast alternative remedies. Table 5-4 lists several remediation technologies that might be compared to surfactant/cosolvent flushing when conducting such an analysis. It should be noted that none of the in-situ technologies listed has successfully restored an aquifer to typical drinking water standards where appreciable quantities of DNAPL are present below the watertable. However, there are cases where these technologies have been successful in achieving regulatory closure at a site. In Table 5-4, the status of the listed technologies is described as either emerging or proven. Emerging refers to a technology that is still in the research stage and has not yet undergone extensive field testing. Proven refers to a technology that has been implemented in the field on many occasions. Proven technologies are not necessarily successful in reaching typical concentration-based cleanup standards.
The criteria used in conducting comparisons between various technologies could include those prescribed in EPA guidance documents (USEPA, 1993). The criteria typically include, among other things, implementability, effectiveness, duration, and cost. These are discussed below.
Implementability
Table 5-5 presents a qualitative ranking of how site conditions impact the complexity of implementing a surfactant/cosolvent flushing project. In general, high-permeability settings are favored because the technology typically requires cycling numerous fluid pore volumes through the target area. Layered systems are considered more challenging because flow along preferred paths will limit contact in less-permeable intervals. Fractured rock is considered the most challenging physical setting due to the complexity of flow in fractured media.
Surfactant/cosolvent flushing systems are most commonly considered for NAPL contaminants. LNAPLs may be easier to remove than DNAPLs because they typically remain concentrated near the top of the watertable and are more likely to form relatively continuous pools. DNAPL removal will likely be more challenging due to concerns about downward mobilization and a typically more complex and sparse distribution beneath the watertable. Secondarily, surfactant/cosolvent systems may be applicable for in-situ recovery of sorbed contaminants. An example of this is the Picatinny Arsenal site described in Appendix A. Surfactant/cosolvent flushing generally is not considered applicable where dissolved compounds are the target because these are already dissolved in the aqueous phase.
It is important to note that challenges imposed by increasing geologic complexity and DNAPLs affect all in-situ remediation technologies. Therefore, the relative merits of surfactant/cosolvent flushing in complex settings need to be compared to what other technologies may be able to achieve in the same settings. Such a comparison may indicate that surfactant/cosolvent flushing can remove contaminant mass from the subsurface much faster than other technologies.
|
Table 5-4 |
|
Alternative Remediation Technologies |
| |
Name
(Status) |
Description |
Short/Long Term |
Above/Below Watertable |
|---|
| |
Steam Flooding (emerging)
|
Inject steam to volatilize high vapor pressure compounds.
|
Short - generally less than 2 pore volumes of steam utilized
|
Above and below |
|
Excavation (proven)
|
Excavate contaminated soils and treat on site, or ship off site for disposal
|
Short
|
Above, with application below watertable if dewatering is feasible |
|
Pump-and-Treat (proven)
|
Construct pumping wells to hydraulically capture dissolved plume derived from NAPL sources
|
Long - generally needs to be implemented for several decades
|
Below |
|
Soil Vacuum Extraction (proven)
|
Extract air to enhance vaporization of NAPL and volatilization of dissolved contaminants
|
Short - time scale highly dependent on formation permeability
|
Above, with application below watertable if groundwater depression feasible |
|
Physical Interception or Hydraulic Containment (proven)
|
Construct sheet pile, slurry, or similar barrier around NAPL zone and/or hydraulically contain with wells or drain lines
|
Long - does not reduce contaminant mass in subsurface
|
Above and below |
|
Permeable Iron Walls (emerging)
|
Construct trench filled with granular iron. Iron degrades certain contaminants in aqueous phase.
|
Long - does not accelerate rate of NAPL dissolution into groundwater
|
Below |
|
Air Sparging (emerging)
|
Inject air below watertable to volatilize dissolved VOCs
|
Long - limited by diffusion towards flowing air pathways
|
Below |
|
Bioventing (proven)
|
Inject air above water-table to enhance biodegradation
|
Long - limited by biodegradation rates
|
Above, with application below watertable if groundwater depression feasible |
|
Biodegradation (emerging)
|
Degrade contaminants either naturally, or enhanced by injecting nutrients, etc. For chlorinated solvents, organics (e.g., acetate) may be added
|
Long - limited by rate of NAPL dissolution into groundwater
|
Below |
|
Natural Attenuation (emerging)
|
Allow dissolved plume to attenuate naturally through both degradation and dispersion processes
|
Long - does not accelerate rate of NAPL dissolution into groundwater
|
Above and below |
|
Waterflooding (proven)
|
Inject/withdraw water to manipulate capillary pressures to induce NAPL migration towards recovery wells/drains
|
Short
|
Below |
|
|
Table 5-5
|
| Relative Applicability of Surfactant/Cosolvent Flushing |
|
|
Contaminant Type or Phase |
|
Hydrogeology |
LNAPL
|
DNAPL
|
Sorbed
|
Aqueous Dissolved |
|---|
|
|
Homogeneous
|
|
|
|
|
|
High Permeability
|
1
|
2
|
2
|
NA |
|
Moderate Permeability
|
2
|
3
|
3
|
NA |
|
Low Permeability
|
3
|
4
|
4
|
NA |
|
Heterogeneous
|
|
|
|
|
|---|
|
Moderate Contrasts
|
2
|
3
|
3
|
NA |
|
Large Contrasts
|
3
|
4
|
4
|
NA |
|
Fractured Rock
|
4
|
5
|
5
|
NA |
|---|
Note: 1 is the most favorable and 5 the least favorable; NA = Not Applicable.
|
In addition to the below-ground challenges to the implementation of surfactant/cosolvent technologies, operation of the above-ground systems also may be somewhat complex. In general, surfactant/cosolvent systems have a moderate to low score for implementability. However, other technologies for removing pooled and residual NAPL may be even more complex and difficult to implement.
Effectiveness
Effectiveness is defined as the ability of a technology to achieve the desired remedial objective at a particular site. The ability of any remediation system to achieve the remedial objectives will be very site specific. Results from previous studies provide a general idea of the effectiveness of surfactant/cosolvent systems. Chapter 2 summarizes some of these systems.
Duration
As discussed in Section 5.1.5, the duration of a surfactant/cosolvent application will be a function of the hydraulic conductivity of the target contaminated zone and surrounding formation and the number of pore volumes needed for the type of surfactant/cosolvent system selected. For example, the duration of treatment will be much shorter for systems requiring only a few pore volumes (e.g., mobilization systems) than for those requiring many pore volumes (e.g., solubilization systems). The time required to complete laboratory and field studies also should be taken into account when estimating the total duration of a surfactant/cosolvent application.
Cost
Finally, the economic feasibility of any remediation technology must be considered. A technology must be reasonably cost-effective to warrant further evaluation. At this stage, order-of-magnitude cost estimates can be prepared using standard engineering practices. Chapter 6 of this manual discusses costs for surfactant/cosolvent systems.
5.2 Additional Site Characterization
Relevance
Typically, site characterization is a phased process that begins with initial site investigations and continues through remedial design and operations. Through each of these phases, data needs arise as the site conceptual model and project objectives evolve. By the time specific corrective actions are seriously evaluated, the type and distribution of contaminants have generally been studied. With these assumptions as a starting point, the following sections describe additional site characterization efforts that would be useful in evaluating and implementing a surfactant/cosolvent remedy. Readers planning to implement a surfactant/cosolvent flushing project should read this section, while those only interested in a preliminary evaluation of the technology may skip this section.
Key Points
• Delineating the horizontal and vertical extent of the NAPL target zone, and estimating the volume of NAPL in place, is a necessary prerequisite to successful field implementation of a surfactant/cosolvent flood.
• This is achieved through a variety of methods, including identification of areas of historical NAPL use, direct observation in boreholes and soil samples, inferences based on groundwater concentrations, calculations of bulk retention capacity, consideration of soil concentrations, and the use of partitioning tracers.
• Even if all of the above methods are employed, a high degree of uncertainty may still exist in the estimate of initial NAPL volume in place.
• Water, NAPL, and soil samples need to be collected to support laboratory design of the surfactant/cosolvent flushing system.
5.2.1 Delineating the Horizontal and Vertical Extent of NAPL
Estimating the extent of NAPL target zones at a site and the volume of NAPL in those zones (both pooled and residual) is often the most difficult aspect of site characterization. This is particularly true for DNAPLs, and in particular chlorinated solvents, which typically migrate in tortuous patterns, forming sparsely distributed pools and zones of residual. It is important to note that locating individual NAPL pools and zones of residual is not feasible at most sites, nor is it of any benefit. An overall region of the subsurface within which NAPL pools and residual exist should be delineated. It is this overall NAPL zone through which surfactants/cosolvents should be flushed. Building on Cohen and Mercer (1993), an iterative approach to estimating the horizontal and vertical NAPL boundaries, as well as the volume of NAPL within those boundaries, is presented below. Because the methods presented here may yield estimates that vary by a factor of 10 or more, some experience an
d judgment will be required to arrive at a best estimate of NAPL volume and spatial extent once each of these methods has been evaluated.
Areas of Historical Use
As a first cut, areas where NAPLs may potentially be present can be approximated by identifying locations where NAPLs were historically stored, used, or disposed. Examples include product storage tanks, buried transmission piping, sumps, floor drains, and disposal pits. Coupling this information with an understanding of subsurface stratigraphy and LNAPL/DNAPL transport processes should provide a good first estimate of NAPL target zones. It should be noted, however, that NAPL typically spreads laterally as it migrates downward, so that NAPL will likely exist outside of the areas of historical use.
Direct Observation
In some instances, the NAPL target zone can be identified through direct observation. Direct observation may include visual observation of NAPL in soil or rock cores, visual observation of NAPL in surface seeps, and/or measured thicknesses of NAPL in monitoring wells. Where NAPL is encountered in wells, it is important to note that the observed thickness in the wells will not correspond to an equivalent thickness in the formation. In fact, the relationship between NAPL in wells and NAPL in the formation is a complex function of fluid properties, sediment characteristics, and historical watertable fluctuations. Many of the details of these interactions are covered in Marinelli and Durnford (1996).
Dissolved Concentrations
Often, NAPLs are present at a site but are not observed directly for a number of reasons: NAPL distribution within the NAPL zone may be sparse, some NAPLs are colorless, and residual NAPL will not flow to a well. Under these conditions, a rough estimate of NAPL extent can be drawn from water quality data. As a rule of thumb, if dissolved concentrations are at or above 1 percent of effective solubility, it is likely that the well is completed in the vicinity of a NAPL zone. This effective solubility relationship for dissolved constituents in NAPL zones reflects slow rates of mass transfer from NAPL bodies to the aqueous phase, hydrodynamic dispersion, and borehole dilution. Using the 1-percent rule of thumb, concentrations that indicate the likely presence of some common single-component NAPLs are presented in Table 5-6.
|
Table 5-6
|
|
Single-Component Concentrations Potentially Indicative of NAPL
|
Compound
|
One Percent of Single
Compound Solubility
(mg/L) |
|
|
Carbon Tetrachloride (CTET)
|
7.6 |
|
1,2-Dicloroethane (DCA)
|
85 |
|
1,2-Dichloroethene(cis) (DCE)
|
35 |
|
Methylene Chloride
|
200 |
|
Tetrachloroethene (PERC)
|
1.5 |
|
Trichloroethane (TCA)
|
15 |
|
Trichloroethene (TCE)
|
11 |
|
Benzene
|
18 |
|
Toluene
|
5.4 |
|
If the NAPL is actually a mixture of compounds, such as petroleum fuels, creosote-based wood-treating oils, coal oil, or mixed solvents, the effective solubility of individual compounds is a function of the compound's mole fraction in the NAPL and its pure-phase solubility. This relationship is described through Raoult's Law as:
Ci = XiSi (5)
where Ci is the effective solubility of component i, Xi is the mole fraction of component i in the NAPL phase, and Si is the single-component or "textbook" aqueous solubility of component i. Using the above relationship requires a knowledge of the primary NAPL components. This can be obtained by collecting and analyzing a representative set of NAPL samples for the individual compounds. A second method is to use the dissolved phase concentrations in groundwater and apportion the mole fractions in the same ratio. The second approach may show a bias if any of the primary compounds are naturally attenuated through either biological or physical processes. Application of Raoult’s Law is described in detail in Feenstra (1990) and Pankow and Cherry (1996).
Table 5-7 provides a summary of dissolved concentrations detected in wells completed through actual, multicomponent NAPL zones at various types of industrial sites. Site-specific concentrations that indicate the presence of NAPL can be verified by measuring dissolved concentrations in wells completed in known NAPL zones (e.g., where NAPL was directly observed) and using those concentrations as a threshold for other areas with similar soils and well completions.
|
Table 5-7
|
|
Dissolved Concentration Typically Observed in Multicomponent NAPL Zones
|
Type of Site
|
NAPL
|
Parameter
|
Typical Dissolved Concentration Range (mg/L) |
|
|
Wood-Treating
|
Creosote Based Wood-Treating Oils (DNAPL)
|
Total Polycyclic Aromatics Hydrocarbons
|
1-10 |
|
Manufactured Gas
|
Coal Tar (DNAPL)
|
Total Polycyclic Aromatics Hydrocarbons
|
1-10 |
|
Fuel Production/Storage
|
Gasoline (LNAPL)
|
BTEX
|
1-10 |
|
Diesel (LNAPL)
|
BTEX
|
|
|
Jet Fuel (LNAPL)
|
BTEX
|
1-10 |
|
Natural Gas Condensate (LNAPL)
|
BTEX
|
1-10 |
|
Chlorinated Solvent Storage/Disposal
|
Chlorinated Solvents (DNAPL)
|
Total Volatile Organic Compounds
|
1-10 |
|
|
|
|
|
Bulk Retention Capacity Calculations
A rough estimate of the spatial extent of NAPL can be made if an estimate of the volume released is available. In order to carry out this calculation, the shape of the NAPL zone must be assumed. A bulk retention capacity must also be assigned to the porous medium in question. The volume of NAPL released is related to the bulk retention capacity through the following expression:
Br = VN/VT (6)
where Br is the bulk retention capacity of the porous medium for NAPL, VN is the volume of NAPL that was released, and VT is the total volume of the overall zone containing residual and pooled NAPL. This overall zone includes both lenses and laminations containing NAPL, as well as interspersed lenses and laminations that are void of NAPL. As discussed in Chapter 3, values of Br may range from 0.25 to 3 percent.
Consider, for example, the release of 1,893 L (500 gal) of NAPL into a rectangular volume having a width W, a length L, and a depth D. The depth of the NAPL zone can be calculated as:
D = VN/(LWBr) (7)
If the bulk retention capacity is 1 percent, and it is known that L and W are equal to 9.15 m (30 ft) and 6.10 m (20 ft), respectively, the total depth of the NAPL zone would be 3.39 m (11.1 ft). If L and W were equal to 3.05 m (10 ft) and 1.52 m (5 ft) respectively, the depth of the NAPL zone would be 40.83 m (134 ft). The above equations can also be used to estimate the volume of NAPL released (VN) if independent estimates of the shape of the NAPL zone can be obtained using other methods such as soil sampling and groundwater concentration data.
Soil Cores
Soil cores are typically obtained using equipment such as hollow stem augers and direct push tools. Careful visual observations of sediment type and NAPL occurrence are recorded along with analysis of sediments potentially containing NAPL. Care must be taken, however, when physically intruding a DNAPL source zone to ensure that capillary barriers supporting DNAPL pools are not punctured. It is becoming increasingly common to implement a DNAPL contingency plan at sites when drilling or coring in suspected source zones. Such plans outline a set of responses to be taken if mobile DNAPL is encountered. One typical response might be to stop drilling when mobile DNAPL is encountered, install a sump to assist in the collection of mobile DNAPL, and chose an alternative location to complete the boring in question.
During coring operations, a variety of field screening methods should be used to assess the presence of NAPL. Table 5-8 summarizes some of these field screening methods. A number of subsamples should be taken from the cores and submitted to a laboratory for soil analysis. In the case of volatile organic samples, consideration should be given to preserving the subsamples in methanol as described in the EPA’s Contract Laboratory Protocols. This is based on the potential to deplete NAPL in soil through volatilization from the subsamples. A rigorous review of the methods used to perform this sampling can be found in Cohen and Mercer (1993).
|
Table 5-8
|
|
Field Method for Screening Samples for NAPLs
|
|
|
Field Screening
Method
|
Comments |
|
|
Visual Inspection
|
Some LNAPLs, DNAPLs, Wood-Treating Oils, and Coal Tars can be visually observed where present in sediments and monitoring wells. |
|
Measurement of Organic Vapors in Sample Head Space
|
Samples containing volatile NAPLs often yield high levels of volatile organic compounds (VOCs). Numerous types of field organic vapor analyzers are available and are typically required as a component of a NAPL site Health and Safety Plan. |
|
Hydrophobic Dyes
|
Where the NAPL of concern is colorless, a hydrophobic dye may be added to the sediment sample. Observation of the dye in liquids indicates NAPL presence. |
|
Ultra Violet (UV) Fluorescence
|
Organic compounds with conjugate double bonds (e.g., aromatic hydrocarbons) fluoresce in the presence of UV light. Cohen and Mercer (1993) report this is also true for many chlorinated solvents. Fluorescence can be verified by testing a representative NAPL sample. |
|
Centrifuging Sample
|
It is possible to separate liquids, including NAPLs, from field sediment using a field or laboratory centrifuge. |
|
|
Once the core subsamples have been analyzed by a laboratory, the data will report contaminant concentrations on a mass per mass basis (i.e., mass of contaminant per unit mass of dry soil). At a coarse screening level, soil concentrations of 1,000 to 2,000 mg/kg equate to approximately 1 percent NAPL saturation in a matrix with 25 percent porosity and NAPL densities ranging between 0.8 and 1.6 gm/cm3. Taking a more refined approach, the maximum concentration in soil in the absence of NAPL can be estimated as described below. Concentrations exceeding this threshold can be considered to be indicators of NAPL presence. The method presented here assumes equilibrium partitioning and follows the procedure outlined in USEPA (1992). Further details can be found in various sources such as Feenstra, et al. (1991).
For soil samples taken from below the watertable, the total soil concentration must exceed the following in order to infer that NAPL was present in the sample:
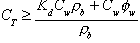 (8)
(8)
where
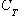 is the total soil concentration (mg/kg),
is the total soil concentration (mg/kg),
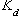 is the soil - water distribution coefficient (ml/g),
is the soil - water distribution coefficient (ml/g),
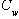 is the effective aqueous solubility of the compound (mg/L),
is the effective aqueous solubility of the compound (mg/L),
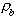 is the dry bulk density of the soil (g/cc), and
is the dry bulk density of the soil (g/cc), and
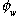 is the water-filled porosity (dimensionless). For soil samples taken from above the watertable, the presence of the air phase must be accounted for as follows:
is the water-filled porosity (dimensionless). For soil samples taken from above the watertable, the presence of the air phase must be accounted for as follows:
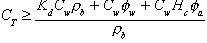 (9)
(9)
where
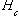 is the dimensionless Henry's constant, and
is the dimensionless Henry's constant, and
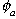 is the air-filled porosity dimensionless).
is the air-filled porosity dimensionless).
Equations (8) and (9) assume ideal behavior and equilibrium partitioning. The distribution coefficient,
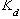 , can be estimated using
, can be estimated using
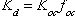 where
where
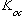 is the organic carbon - water partition coefficient and
is the organic carbon - water partition coefficient and
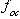 is the fraction organic carbon. For a multicomponent NAPL composed of structurally similar compounds, the effective solubility can be estimated using Raoult's law (Banerjee, 1984). For a single-component NAPL, the effective solubility will equal the "textbook" solubility as reported in handbooks.
is the fraction organic carbon. For a multicomponent NAPL composed of structurally similar compounds, the effective solubility can be estimated using Raoult's law (Banerjee, 1984). For a single-component NAPL, the effective solubility will equal the "textbook" solubility as reported in handbooks.
Table 5-9 presents a variety of example calculations using Equations (8) and (9). In thesecalculations, a porosity of 0.35 is assumed. An organic carbon fraction of 0.003 is used because this would not be uncommon for typical deposits.
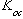 values are taken from Schwille (1988). For the interested reader, a sensitivity analysis can be carried out by specifying values other than these and repeating the calculations. It is clear from Table 5-9 that the threshold concentrations corresponding to NAPL presence can vary from site to site depending on specific geological conditions and whether or not the contaminant of interest is present as a single-component NAPL or part of a multicomponent NAPL.
values are taken from Schwille (1988). For the interested reader, a sensitivity analysis can be carried out by specifying values other than these and repeating the calculations. It is clear from Table 5-9 that the threshold concentrations corresponding to NAPL presence can vary from site to site depending on specific geological conditions and whether or not the contaminant of interest is present as a single-component NAPL or part of a multicomponent NAPL.
Laser-Induced Fluorescence and Cone Penetrometer Testing
Laser-induced Fluorescence (LIF) is an in-situ method of spectroscopy that can be combined with Cone Penetrometer Testing (CPT) to produce continuous recordings of the stratigraph and aromatic hydrocarbon contamination in the subsurface. CPT methods have been used Table 5-9
for many years to determine subsurface stratigraphy. The combination of CPT with LIF is a relatively recent development that was originally developed in part by the Navy. LIF systems utilize a fiber optice/laser/sensor assembly that emits a laser of a specific wavelength through a sapphire window in a CPT cone. When the laser encounters aromatic hydrocarbons, the contaminants fluoresce, which is measured by the sensors. Data are generated continuously while the CPT is advanced at a rate of about 2 feet per second.
LIF/CPT can aid in the delineation of NAPL contamination if the NAPL contains compounds that fluoresce with a laser (e.g., aromatic hydrocarbons). Because LIF/CPT is relatively quick, it may be possible to install a relatively large number of CPT holes (compared to conventional drilling) to delineate the horizontal extent of the NAPL. LIF/CPT should be able to delineate the vertical distribution of the NAPL; however, it is not clear if LIF/CPT is capable of defining the mass or volume of NAPL present in the subsurface.
Partitioning Tracers
In the case of chlorinated solvents, it is widely recognized that actually locating and defining the spatial extent of all the NAPL by the methods described above may be very difficult. An innovation in estimating in-place NAPL volume involves the use of a partitioning tracer test(Jin, et al., 1995).
In the partitioning tracer test, two tracers are injected simultaneously. One tracer partitions into the NAPL while the second nonpartitioning tracer is transported independent of the NAPL. Tracers are recovered from an adjacent recovery well, and concentrations are measured as a function of time and flow rate. The degree of chromatographic separation between the peaks of the tracers is a function of the field-scale bulk average NAPL saturation in the targeted interval. Using the method of first moment analysis, it is possible to estimate the volume of NAPL present (Jin, et al., 1995).
Annable, et al. (1994) describe the use of partitioning tracers at Hill AFB in Utah. Their results indicated that the NAPL saturation was 0.065 (fraction of pore space). This number compares closely to the value obtained through analysis of soil samples, 0.07. The benefits cited by Annable, et al. (1994) include a spatially integrated measurement as opposed to point values obtained through coring, and less intrusive sampling that may prevent inadvertent vertical migration. The tracers used in this study include bromide (applied as KBr), ethanol, n-pentanol, n-hexanol, and 2,2-dimethyl-3-pentanol.
Potential limitations of the partitioning tracer method include the following:
•Partitioning to natural organic carbon may make it difficult to identify low NAPL saturations.
•Contaminants in areas not swept due to heterogeneity and/or nonuniform flow patterns may not be detected.
•Thick pools of mobile NAPL will be subject to mass limitations, rendering estimates below the actual amount present.
•Costs associated with defining tracer concentration over time may be high.
•Biological attenuation may impact the mobility of the organic tracers.
Despite these concerns, the partitioning tracer method holds promise. The largest benefit in applying this test may not be in the estimated NAPL volume, but in a better understanding of the overall size of the NAPL zone to be remediated.
Refinement of Estimates
At most sites, the estimate of NAPL volume in place will be refined with time as additional characterization data become available. Once pilot or full-scale recovery activities have been initiated, further refinements can be made to the estimate by using cumulative production data to determine the minimum amount of NAPL that may be present.Based on work by Frick and Taylor (1962), this process is illustrated in Figure 5-7. The concept is based on the fact that the cumulative volume of NAPL produced in time will reach a well-defined asymptote.
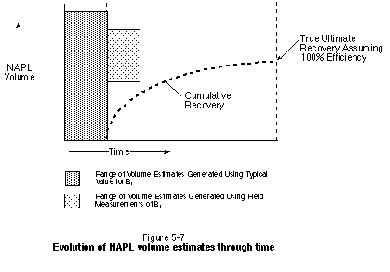
As an example, approximately 1,600 shallow test holes were drilled at a former wood-treating site in Laramie, Wyoming, as part of a full-scale (60-acre) waterflood mobile DNAPL recovery project (Sale and Applegate, 1996).Initially, a correlation to measured DNAPL content was developed for visually logged mobile and residual DNAPL saturations. The observed thicknesses of mobile DNAPL in cores were then used to estimate total mobile DNAPL volume. Finally, waterflood DNAPL recovery was conducted until asymptotic production curves indicated 95 percent or more of the mobile DNAPL had been recovered. The validity of using production curves to document 95-percent recovery of mobile DNAPL was verified through analysis of post-recovery soil samples. Comparisons of estimated mobile DNAPL volumes to recovered volumes from the 50 or so recovery cells indicated that this approach to estimating mobile DNAPL volume was accurate to within a factor of two (Sale and Applegate, 1996).
At the same site, as part of a large-scale pilot of alkaline-polymer-surfactant flooding, 18 soil cores were collected before and after chemical flushing in a 40-m by 40-m test cell. The mass balance developed using the soil core analyses suggested 67,000 L (17,600 gal) of DNAPL had been recovered, while produced fluids data indicated 89,000 L (23,600 gal) had been recovered (Sale and Applegate, 1996).
5.2.2 Collecting NAPL, Water, and Matrix Samples for Laboratory Studies
Fundamental to assessing the feasibility of surfactant/cosolvent flushing is quantifying the physical properties of the soils, groundwater, and NAPL. Collection of representative samples for laboratory analysis is a critical aspect of site characterization. Table 5-10 lists samples that are typically collected for laboratory work and estimates of the sample volume or mass required. The types of tests to be performed on these samples are discussed further in Section 5.3.
|
Table 5-10
|
|
Field Samples Needed for Laboratory Studies
|
|
|
Sample
|
Amount Required |
|
|
NAPL
|
Approximately 1 L is needed for fluid characterization. An additional 5 to 20 L are needed for bench-scale testing of chemical systems (e.g., column tests). |
|
Groundwater
|
Approximately 1-2 L are required for fluid characterization. An additional 5 to 20 L are needed for bench-scale testing of chemical systems (e.g., column tests). |
|
Soil or Rock
|
Sufficient material needs to be collected to characterize the range of conditions. For a single sediment type, a minimum of 2 kg should be obtained. To the degree possible, intact sediment samples should be collected. |
|
|
|
5.3 Laboratory Studies
Relevance
Laboratory studies are an essential component in the design of successful surfactantcosolvent floods. Laboratory studies are typically carried out prior to field-scale demonstrations, and are generally conducted in a university or specialty consulting laboratory. It is important to realize that each site requires a tailored design and that simply taking a surfactant formulation that was successful at one site and applying it to another site will most likely lead to failure.
Readers who have experience in designing surfactant/cosolvent floods at waste sites may choose to skip this section. Similarly, readers seeking information with which to decide whether a surfactant/cosolvent flood is appropriate for a particular site may skip this section.
Key Concepts
• The contaminant type, soil characteristics, geology, and groundwater geochemistry will vary from site to site, warranting site-specific laboratory studies.
• If a selected chemical system is not successful in the laboratory, it will likely fail in the field.
• The first phase of laboratory studies generally includes an initial screening for surfactantcosolvent selection, NAPL characterization, groundwater characterization, and soil characterization.
• The second phase of laboratory studies generally includes a series of batch tests to evaluate phase behavior, sorption characteristics, solubilization potential, and interfacial tension alteration.
• The third phase of laboratory studies generally includes a series of one-dimensional column experiments and two-dimensional sand-pack experiments to evaluate cation exchange capacity, permeability alteration, relative permeability effects, rate-limited mass transfer, degree of mass removal, surfactant degradation, hydrodynamic instabilities, and the use of polymers.
• Biodegradability testing of the surfactants or cosolvents used may be required if little information exists on their potential fate in the environment.
• Produced fluids treatment can include gravity separation, dissolved-air floatation, chemical demulsification, acid treatment, heat treatment, air stripping, steam stripping, membrane separation, biological treatment, solvent extraction, pervaporation, oxidation, and addition of absorption media.
• Bench-scale testing of produced fluids treatment will depend on the particular treatment method used.
5.3.1 The Need for Laboratory Studies
After additional site characterization is completed, and results indicate that surfactant/cosolvent flushing may be a feasible and favored alternative for the site, laboratory studies should be undertaken to select or design the specific chemical blend that will effectively remove NAPL from the contaminated soil. The contaminant type, matrix characteristics, and groundwater geochemistry are unique for each site, so that a site-specific laboratory program will be necessary to design an effective surfactant/cosolvent system.
Laboratory studies also will provide information on the potential field performance of a surfactantcosolvent flushing system. While laboratory studies will not ensure that NAPL can be efficiently removed by surfactant/cosolvent flushing at the site, if a chemical system does not remove NAPL from soil samples in the laboratory, the chemical system will likely not effectively remove NAPL contamination from soil at the site.
Laboratory studies also should include evaluations of treatment and handling of the produced fluids generated from a surfactant/cosolvent flood. Data from the produced fluids treatment testing should be taken into account in the chemical design studies (e.g., formulations may be changed so that the surfactant can be reused) to optimize the overall process.
No standard protocol exists for laboratory studies of surfactant-based in-situ soil flushing. However, it is generally acknowledged that certain parameters should be identified and certain tests should be performed before attempting a field project. The remainder of this section describes laboratory screening tests and methods for assessing the effectiveness of chemical systems. It is assumed that the reader is familiar with the concepts and terminology presented in Chapter 4.
5.3.2 Initial Surfactant/Cosolvent Screening and Selection
Screening and selection of appropriate surfactants and cosolvents should take into account site-specific information as well as the characteristics of the particular surfactant/cosolvent. There are hundreds of different surfactants available. They can generally be grouped into one of four categories: anionic, nonionic, cationic, and zwitterionic. Generally, only anionic and nonionic surfactants are technically and publicly acceptable for soil flushing. Even within these two categories, there are hundreds of surfactant structures derived from synthetic and natural sources. Factors to consider in screening and selecting surfactants and cosolvents include:
• Prior application experience
• Potential effectiveness for this application
• Cost
• Public and regulatory perception
• Biodegradability and degradation products
• Toxicity to humans, animals, and plants
• Ability to treat/handle resulting produced fluids (ideally, the surfactant/cosolvent can be reused)
The performance of a surfactant or cosolvent at other similar sites should be investigated first. Table 5-12 lists some of the surfactants that have been studied for environmental remediation projects. It should be noted that the types of surfactants being used currently are somewhat different from those used a few years ago because of changes in surfactant chemistry and concerns about the toxicity of earlier surfactants. Appendix A provides additional summary information related to the projects listed in Table 5-12.
Selected surfactants should have low potential for sorption by the soil. The efficiency of NAPL removal may decrease as chemical is lost from solution and sorbed on the soil. Generally, anionic surfactants will adsorb at a lower rate than nonionic surfactants, which in turn adsorb at a lower rate than cationic surfactants. The initial screening of alcohols generally does not take into account sorption characteristics because alcohols typically do not adsorb onto aquifer solids.
To reduce concerns of regulators and the public, researchers have investigated "edible" surfactants (Sabitini, et al., 1995) and "FDA approved" surfactants. However, being FDA approved or even edible does not guarantee that the compound or its biodegradation products are safe and that the regulators and the public will accept them. The same surfactant type sold under a different trade name may not be FDA approved for the process. For one pilot test of the surfactant-enhanced solubilization of TCE, the state refused to allow injection of the technically most viable surfactant because it could contain unreacted reagent chemicals that are prohibited in that state’s groundwaters, even though the surfactant had a toxicity similar to that of sucrose and was classified for use in food and for food processing (Fountain, et al., 1996).
Different surfactant types and structures represent a wide range of toxicity to animals and plants. The ingestion hazard to humans of many anionic or nonionic surfactants is low (e.g., swallowing residual dish soap is not harmful and ingestion of larger amounts may result only in diarrhea). However, some of these surfactants may be hazardous to aquatic life. For example, ethoxylated alcohols (which have been used in remediation projects and are common in detergent formulations, including dish soap and laundry detergent) are toxic to fish, but are not a significant environmental concern when used as household detergents because they readily degrade in biological wastewater treatment systems. The biodegradation of surfactants is discussed further in Chapter 4.
|
Table 5-12
|
|
Surfactants Used for Remediation Field Projects and Laboratory Studies
|
|
|
Project
|
Specific Chemicals |
|
|
Laramie, Wyoming - Small Field Demonstration
|
Sodium Dodecyl Benzene Sulfonate, Ethoxylate Nonylphenol, NaHCO3,Na2CO3, and Xanthan Gum |
|
Warren, Michigan
|
Nonionic Ethoxylated Alcohol |
|
Laramie, Wyoming - Large Field Demonstration
|
1.4 wt % Ethoxylateted Nonylphenol, 0.72 wt % NaHCO3, 1.0 wt % Na2CO3, and 1050 mg/L Xanthan Gum |
|
Hialeah County, Florida
|
0.5 wt% Na2CO3, 1.1 wt% NaHCO3, 0.5 wt% Na2O(SiO2), Chloramine T, 1000 mg/L Xanthan Gum |
|
Canadian Forces Base Borden, Ontario
|
1 wt% phosphate ester of nonylphenol ethoxylate |
|
Fredricksburg, Virginia
|
0.5 wt% Na2CO3, 0.1 wt% Nonylphenol with 10 moles EO, 1500 mg/L Xantham Gum |
|
Corpus Christi, Texas
|
Not Known at This Time |
|
Quebec City, Quebec
|
Not Known at this Time |
|
Paducah, Kentucky
|
1 wt% Sorbitan Monooleate |
|
L'Assomption, Quebec
|
__wt% n-Butanol, __ wt% Hostapur SAS (Hoechst GmbH), __ wt% Toluene, __ wt%d-Limonene |
|
Westmoreland County, Pennsylvania
|
Ethoxylated Alcohol and Cocamidopropyl Betaine |
|
Commercial Site, New Jersey
|
Not Known at This Time |
|
Hill AFB, Utah - Ethanol OU#1
|
70% Ethanol Plus 12% n-pentanol |
|
Picatinny Arsenal, New Jersey
|
Not Known at This Time |
|
Traverse City, Michigan
|
Dowfax 8390 |
|
Hill AFB, Utah - Cyclodextrin Solubilization, OU1 Cell #4
|
Hydroxypropyl-ß-Cyclodextrin (HPCD) |
|
Hill AFB, Utah - Surfactant Solubilization, OU1, Cell #5
|
DOWFAX 8390 |
|
Hill AFB, Utah - Surfactant Mobilization, OU1, Cell #6
|
Aerosol OT and SMDNS |
|
Hill AFB, Utah - Surfactant with Cosolvent, OU1 Cell #8
|
3.5 wt% Brij 91[Polyoxyethylene (10) Oleyl Ether] and 2.5 wt% n-pentanol |
|
Hill AFB, Utah - OU2 Micellar Flood
|
Sodium Dihexyl Sulfosuccinate (7.5 wt%), Isopropyl Alcohol (3.75 wt%), and 7000 mg/L Sodium Chloride |
|
Hill AFB, Utah - OU1 Cell #3
|
Tert-Butanol/Hexanol |
|
Piketon, Ohio
|
4 wt% Sodium Dihexyl Sulfosuccinate, 4 wt% isopropyl alcohol, and a 1:1 mixture NaCl and CaCl2 |
|
Hill AFB, Utah - OU2 Foam Flood
|
Not Known at This Time |
|
Fort Worth, Texas
|
Not Known at This Time |
|
|
|
With respect to costs, surfactants for environmental applications are generally priced between $1.10 and $5.52 per kilogram ($0.50 and $2.50 per pound). Water-miscible alcohols are generally priced between $0.35 and $1.20 per kilogram ($0.16 and $0.54 per pound). In most cases, significant savings can be realized by purchasing bulk quantities. The manufacturer of a particular surfactant or alcohol should always be contacted to obtain up-to-date costs.
5.3.3 NAPL, Water, and Soil Characterization
NAPL Characterization
Before appropriate chemicals for a site can be selected, knowledge of the physical and chemical properties of the NAPL, groundwater, source water, and soil needs to be refined. Some of these properties may not be clearly defined during the site characterization process, and therefore must be determined during initial laboratory screening.
Properties of the NAPL, such as density, viscosity, effective solubility in water, NAPL - water interfacial tension, and component composition are related to the transport of the contaminant through the soil and are therefore critical to an effective flushing system. Table 5-13 lists the NAPL properties that should be measured and the methods used to carry out the measurement. If possible, these properties should be measured at the subsurface temperature of interest. This is particularly true for viscosity, which is known to be a strong function of temperature.
|
Table 5-13
|
|
Methods for NAPL Characterization
|
|
|
Parameter
|
Method Description
|
Method |
|
|
Density
|
Hydrometer
|
1ASTM D 1298 |
|
Pycnometer
|
1ASTM D 1217 |
|
Pycnometer
|
1ASTM D 1480 |
|
Viscosity
|
Capillary (kinematic)
|
1ASTM D445 |
|
Rotational (Brookfield)
|
|
|
Interfacial Tension
|
Ring (DuNouy)
|
1ASTM D971 |
|
Pendant drop (drop weight)
|
1ASTM D2285 |
|
Spinning drop
|
Cayias, et al., 1975 |
|
|
NAPL Composition
|
Volatile organics
Semivolatile organics
Pesticides/PCBs
Total petroleum hydrocarbons
Gasoline range organics
Diesel range organics
|
2SW846-8260
2SW846-8270
2SW846-8080
3EPA418.1, 2SW846-9071
2SW846-8015M
2SW846-8015M |
|
|
NOTES:
1American Society for Testing and Materials, 1995.
2Test Methods for Evaluating Solid Waste, SW-846, 3rd edition. USEPA, November 1986, as amended.
3Methods for Chemical Analyses of Water and Wastes, EPA600/4-79/020. USEPA, March 1983, as amended.
|
Although the tests listed in Table 5-13 can generally be performed with less than 100 ml of sample, it is preferable to collect 500 mL or more. The NAPL sample should be covered with groundwater from the site and placed into a glass jar containing no head-space. If possible, the sample should be shipped to the laboratory under chilled conditions to prevent biological activity at the NAPL - water interface. Testing of samples collected from various wells will give an indication of the spatial variability of the NAPL throughout the site. At a mixed-waste site, such as a solvent recycling facility, multiple types of NAPL may be encountered in the subsurface. At a site that employed only one specific process involving NAPL, such as a dry cleaning facility, there will likely be little spatial variability in the NAPL properties.
At a mixed-waste site, certain components may not be identifiable using state-of-the-art GC/MS analysis. When calculating effective solubilities in water, an estimate of the unknown fraction molecular weight will have to be made to calculate the relative mole fractions of the identified compounds. When doing so, a range of unidentified compound molecular weights should be employed to yield a range of possible effective solubilities. Even when all NAPL components can be identified, the mass balance may not sum to unity because of errors arising from the high dilutions typically required in such analyses. In such cases, the mass fractions can be normalized to unity in order to calculate mole fractions.
Water Characterization
Water characterization is an important design consideration for surfactant/cosolvent soil flushing because the effectiveness of the injected chemical solution can be altered if it is diluted with groundwater having a significantly different ionic composition than the water used in the solution. Some surfactant classes may not be usable for certain groundwater conditions. In addition, flushing with a water of different ionic composition than the groundwater (such as city tap water) may result in precipitation of insoluble minerals from the resulting blend. This precipitation can reduce the soil permeability. Iron fouling also is an important consideration in cycling fluids using water with a high dissolved iron content. High dissolved iron is very often associated with petroleum hydrocarbon contamination.
The tests to characterize the chemical and physical properties of groundwater and injection water are carried out by many laboratories on a routine basis. Table 5-14 lists some of the test methods available.
|
Table 5-14
|
|
Methods for Water Characterization
|
|
|
Parameter
|
Method Description
|
Method |
|
|
Fluid Properties
|
|
|
|
Viscosity
|
Capillary (kinematic)
|
1ASTM D445 |
|
Density
|
Pycnometer
|
1ASTM D1480 |
|
Inorganic Chemistry
|
|
|
|
Calcium
|
Atomic adsorption
|
APHA 301A II (APHA-AWWA-WPCF) |
|
Magnesium
|
Atomic adsorption
|
APHA 301A II |
|
Iron
|
Atomic adsorption
|
APHA 301A II |
|
Potassium
|
Atomic adsorption
|
APHA 317A |
|
Sodium
|
Atomic adsorption
|
APHA 320A |
|
Barium
|
Atomic adsorption
|
APHA 301A II |
|
Strontium
|
Atomic adsorption
|
APHA 321A |
|
Chloride
|
Mercuric nitrate
|
APHA 408 B |
|
Sulfate
|
Turbimetric precipitation
|
APHA 427C |
|
Carbonate and Bicarbonate
|
Alkaline titration
|
APHA 403 |
|
pH
|
Electrode
|
APHA 424 |
|
Conductivity
|
Electrode
|
APHA 205 |
|
Total Dissolved Solids
|
Gravimetric
|
APHA 208
|
|
Organic Chemistry
|
|
|
|
Volatile Organics
Semivolatile Organics
Pesticides/PCBs
Total Petroleum Hydrocarbons
Gasoline Range Organics
Diesel Range Organics
|
Mass Spectrophometric
Mass Spectrophometric
Gas Chromatographic
Infrared
Gas Chromatographic
Gas Chromatographic
|
2SW846-8260
2SW846-8270
2SW846-8080
3EPA418.1
2SW846-8015M
2SW846-8015M |
|
|
NOTES: 1American Society for Testing and Materials, 1995.
2Test Methods for Evaluating Solid Waste, SW-846, 3rd edition. USEPA, November 1986, as amended.
3Methods for Chemical Analyses of Water and Wastes, EPA600/4-79/020. USEPA, March 1983, as amended.
|
Soil Characterization
A variety of parameters are required to properly characterize the soil at a site. Soil is defined here as any porous media occurring at the site. Note that soil is not restricted to the unsaturated zone, as is assumed by some. The parameters that need to be measured include porosity, permeability, grain size distribution, displacement pressure, dry bulk density, fraction organic carbon, cation exchange capacity, and contaminant concentration. Table 5-15 lists the test methods available to measure these properties.
|
Table 5-15 |
|
Methods for Soil Characterization |
| |
Parameter |
Method Description |
Method |
|---|
| |
Physical Properties |
|
Dry bulk density
Grain size distribution
Permeability and porosity
Wettability (capillary moisture retention)
Total organic carbon
Displacement pressure
Cation Exchange Capacity
|
Physical Method
Physical Method
Physical Method
Physical Method
Infrared Carbon Dioxide
Physical Method
Chemical Method |
1ASTM D2937
1ASTM D422
1ASTM D5084
1ASTM D3152
2SW846-9060
----
2SW846-9081 |
| |
Organic Contaminants
Volatile Organics
Semivolatile Organics
Pesticides/PCBs
Total Petroleum Hydrocarbons
Gasoline Range Organics
Diesel Range Organics |
Mass Spectrophometric
Mass Spectrophometric
Gas Chromatographic
Infrared
Gas Chromatographic
Gas Chromatographic
|
2SW846-8260
2SW846-8270
2SW846-8080
3EPA418.1, SW846-9071
2SW846-8015M
2SW846-8015M |
|
|
NOTES: 1American Society for Testing and Materials, 1995.
2Test Methods for Evaluating Solid Waste, SW-846, 3rd edition. USEPA, November 1986, as amended.
3Methods for Chemical Analyses of Water and Wastes, EPA600/4-79/020. USEPA, March 1983, as amended.
|
Valuable information can be gained in laboratory testing of the permeability of several small-scale samples obtained from the site. Numerous permeability measurements allows the calculation of a variance for the permeability distribution. A higher variance in permeability indicates a higher degree of soil heterogeneity. Such an indication of local-scale heterogeneity cannot be gained from pump tests or a limited number of slug tests. A relatively homogeneous aquifer will exhibit a variance in log-transformed permeability of less than approximately 0.5, while a relatively heterogeneous site will exhibit a variance in log-transformed permeability of greater than approximately 1.0. As discussed previously, the applicability and likelihood of success of a surfactant/cosolvent flood is inversely proportional to the degree of heterogeneity at a site. A site displaying a high degree of heterogeneity will likely experience poor sweep efficiency.
The bulk average field-scale permeability must also be evaluated for the site using pump-test and slug-test data. This type of data is usually available from initial remedial investigation work at a site, and often does not need to be evaluated further during surfactant/cosolvent system design. If uncertainty exists regarding the bulk average permeability of the target NAPL zone, however, then additional characterization work will need to be carried out. Knowledge of the bulk permeability of the target zone is essential to predicting the time required to cycle a given number of pore volumes of injected chemical solution through the subsurface. If possible, test pits also should be completed to gain an understanding of geological structure.
The distribution of grain sizes in an aquifer material is useful for determining the clay content of the material and in designing sand packs for injection and withdrawal wells. The dry bulk density of a soil is required to estimate the attenuation of sorbing constituents (see Chapter 3 for calculation method) and to calculate the threshold soil concentrations indicating the presence of NAPL (see Section 5.2.1 for calculation method). The fraction of organic carbon in a soil also is required to estimate the attenuation of sorbing contaminants and to calculate the threshold soil concentrations indicating the presence of NAPL. Alternatives to using the dry bulk density and fraction organic carbon in estimating the sorption characteristics of a soil are to perform batch sorption tests using the actual soil and chemical constituents of interest (Fetter, 1993), to perform laboratory column tests, and to perform field pilot tests.
The displacement pressure of a soil characterizes the capillary resistance it will offer to the flow of a non-wetting fluid such as NAPL. If the displacement pressure is sufficiently high, no NAPL will enter into the material in question. Full characterization of the capillary characteristics of a soil would include not only measurement of the displacement pressure, but measurement of the entire capillary pressure-saturation curve. Methods to measure the capillary pressure-saturation relationship are described by Bear (1972) and Corey (1986). A site exhibiting spatial variability in permeability will also exhibit spatial variability of the displacement pressure. Kueper and Frind (1991) successfully employed a modified version of the Leverett function (Leverett, 1941) to correlate displacement pressure with permeability for a chlorinated solvent DNAPL - water system in fine- to medium-grained sands. Use of a scaling function such as the Leverett function allows the capillary charact
eristics of a site to be defined using a relatively few number of displacement pressure measurements.
Cation exchange capacity is a measure of the number of cations that may be released from the soil as a result of contact with an injected solution. The released hardness may precipitate or complex with the surfactant or other chemicals and render the remedial surfactant solution ineffective (Pope, et al., 1978; Lake and Helfferich, 1978; Hill and Lake, 1978; Hirasaki, 1982). The amount of cation released is a function of the water properties and the cation exchange capacity of the soil. Table 5-16 lists some values published in the literature.
Table 5-16
Cation Exchange Capacity of Various Soils and Rock
Sample |
Cation Exchange Capacity
(meq/ml pore volume)
|
Reference |
|
Tar Springs |
0.019 |
Lake and Helfferich, 1978 |
|
Berea sandstone
|
0.107 - 0.039
|
Lake and Helfferich, 1978 |
|
Berea sandstone
|
0.047
|
Smith, 1978 |
|
Second Wall Creek
|
0.147 - 0.175
|
Smith, 1978 |
|
Berea sandstone
|
0.047
|
Gupta, 1980 |
|
Berea sandstone
|
0.034
|
Potts and Kuehne, 1988 |
|
Whittier
|
0.191 - 0.232
|
Potts and Kuehne, 1988 |
|
Sandy aquifer
|
0.027 - 0.081
|
Appelo, 1994 |
The cation exchange capacity is the maximum amount of cation exchangeable. The actual number of cations that will exchange from the soils depends upon the water composition and the soil - water partition coefficient. The mass action coefficient is derived from the following equilibrium equation for the exchange of clay calcium (Ca) with solution sodium (Na+), where the clay is represented by X:
CaX + 2Na + = Ca +2 + 2NaX (10)
The resulting equilibrium equation defines the partition coefficient (Hill and Lake, 1978):
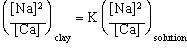 (11)
(11)
The K factor is a property of the soil and varies for each exchangeable ion. Average values for the partition coefficients for particular cations are available from the literature for various soil types or specific clays.
The significance of the cation exchange parameters is that injection of a solution of different ionic makeup than the groundwater that has been in equilibrium with the soil induces cation exchange with the soil that can dramatically affect the solution properties. This is especially true with relatively low-salinity groundwaters in which divalent cations such as calcium and magnesium make up a significant portion of the cationic ion composition. For example, with a groundwater containing 161 mg/L of calcium and 118 mg/L of sodium and having a K value of 0.003, roughly 98 percent of the clays will be in the calcium form. If 1 percent anionic surfactant (with a molecular weight of 400) is injected that has sodium as its cation, the resulting cation exchange with the soil will result in calcium concentrations of more than three times that of the original groundwater calcium concentration until the soil equilibrates.
The sodium/calcium ratio of the solution injected and the cation exchange capacity of the soil also can have a significant effect on permeability of the soils. Dispersion of clays can be caused by high sodium contents. The dispersed clays can plug other pores.
5.3.4 Surfactant/cosolvent Laboratory Screening
Laboratory tests to screen various surfactant systems and to optimize the solutions are relatively rapid and inexpensive. As discussed previously, there are two main mechanisms for NAPL recovery: mobilization and enhanced solubilization. Although these are not entirely independent, surfactants can be selected to emphasize one mechanism over the other. Laboratory screening of surfactants for the two mechanisms are different. Mobilization requires reducing the capillary forces that trap the NAPL in the saturated soil by reducing the interfacial tension. Solubilization consists of incorporating the contaminant in the micelles, requiring that surfactant concentrations be greater than the CMC and that they be maintained as the surfactant flows through the soil.
Laboratory Testing of Mobilization Systems
Once it has been determined whether solubilization or mobilization is most appropriate for the site, an optimized surfactant system can be designed and tested in the laboratory. The optimum system may contain one or more of the following components: surfactant, cosolvent, alcohol, salt, and polymer. These components are blended to enhance the overall performance of the system. The traditional method for optimizing a surfactant system involves adding salt or alcohol. As discussed in Chapter 4, altering the salinity can optimize a surfactant formulation. Salter (1977) and others have demonstrated that the optimal salinity can be identified using either phase behavior tests or interfacial tensions. The interfacial tension between aqueous solutions and NAPL can be measured to screen surfactant systems that significantly reduce capillary trapping forces.
Laboratory testing of interfacial tension also can be used to evaluate the effects of surfactant class and structure, surfactant concentration, salinity and ionic strength, pH, makeup and dilution water effects, and effects of exchanged calcium.
Surfactant solutions can be low concentration (generally the minimum interfacial tension is near the CMC) or high concentration (Chan and Shah, 1981) depending on the type of surfactant used. Figure 5-8, for example, shows the effect of surfactant concentration on the interfacial tension of water and jet fuel. The minimum of interfacial tension shown in the figure is at a surfactant concentration of 0.5 percent.
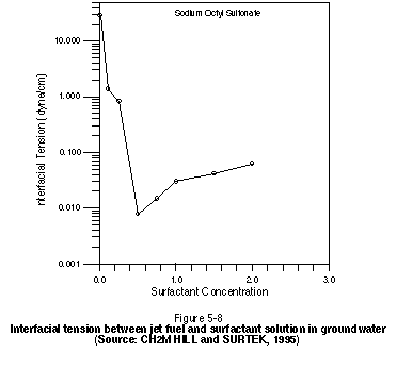
Optimizing a solution by altering the salinity operates by changing the solution’s ionic strength. Figure 5-9, for example, shows the influence of added sodium chloride on NAPL-water interfacial tension. The optimum sodium chloride concentration is defined as the concentration at which the interfacial tension is a minimum. The optimum value is shown to be dependent on the type of water used to prepare the surfactant solution. Different salt types (NaCl, KCl, etc.) or alkaline agents (Na2CO3, NaHCO3, NaOH, etc.) can be used to optimize the surfactant-NAPL interfacial tension.
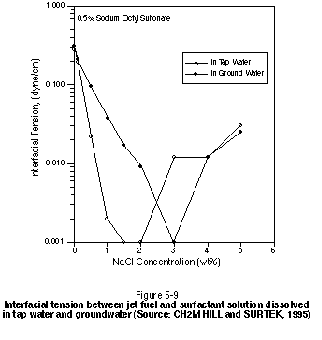
In some cases, NAPL components may react with alkaline agents to form "natural" surfactants, which also can reduce the oil - water interfacial tension. Alkaline agents in combination with surfactants were used in the large-scale field demonstration at the Laramie, Wyoming site (Pitts, et. al, 1993). This phenomenon depends on the solution’s ionic strength and pH (Rudin and Wasan, 1993). Figure 5-10, for example, illustrates the impact of pH on interfacial tension for a solution of constant ionic strength.
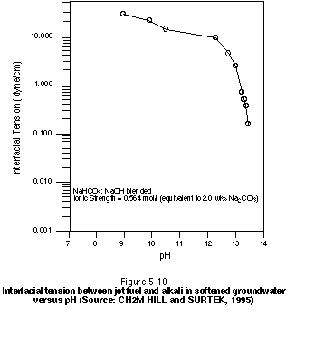
The effects of groundwater dilution on the interfacial tension between the surfactant solution and the NAPL also can be evaluated with interfacial tension tests. For example, Figure 5-11 shows the effect of water type and hardness on the surfactant concentration versus interfacial tension response. Surfactant solutions that have contacted the soil also can be evaluated to determine the effect of the soil cation exchange on the interfacial tension.
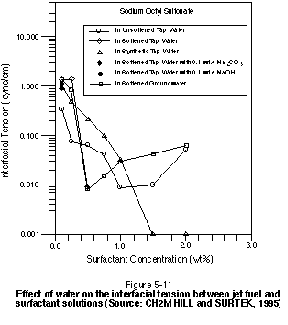
As discussed previously, calcium can affect the performance of some surfactants; this is illustrated in Figure 5-12. Calcium impacts can be handled in several ways. One method is to select a nonionic surfactant that is tolerant of calcium and does not require salinity alteration to achieve desired performance. A second method is to use an optimized solution that minimizes the effect of altering the ratio of (Na+)2/Ca++ in the groundwater so that no calcium is discharged.
Calcium effects also can be addressed by preflushing the target cleanup zone with a saline solution that contains very little calcium in order to discharge any calcium from clay particles. It should be noted, however, that the cation exchange front moves at a lower velocity than the injected water due to the chromatographic effect of ion exchange. The preflush waterflood volume must therefore be substantial enough to prevent interference with the advancing chemical system (Hill and Lake, 1978).
The surfactant solution can be made more tolerant of divalent cations by sequestering them with an EDTA or citric acid. This is demonstrated in Figure 5-13 using citric acid and a 0.75-percent sodium octyl sulfonate solution. As can be seen, the addition of sodium citrate leads to a lowering of NAPL - water interfacial tension. If a sequestering agent is added, however, the solution must be re-optimized with respect to other added chemicals.
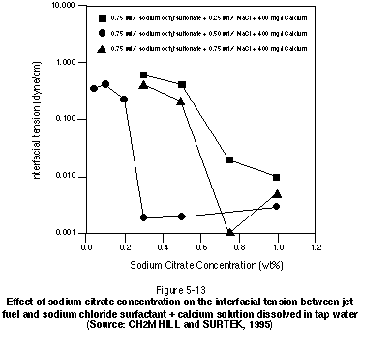
As discussed previously, interfacial tension can be determined using several different methods. The DuNouy ring tensiometer (ASTM D971) measures interfacial tensions greater than approximately 1 dyne/cm and is often used to measure the initial interfacial tension of the NAPL against the groundwater. A spinning drop tensiometer (Cayias, et al., 1975) can measure interfacial tensions less than 1 dyne/cm, the range that is the typical target when designing a mobilization surfactant/cosolvent flood. The spinning drop method can be used only for transparent NAPLs. If the NAPL is opaque, another technique such as the pendant drop method can be used.
Laboratory Testing of Solubilization Systems
This removal mechanism uses highly solubilizing surfactants and cosolvents to incorporate NAPL into micelles. These surfactant systems are selected so as not to reduce interfacial tension to ultra-low levels. The amount of NAPL solubilized generally increases with an increase in the size of the micelle and an increase in the total surfactant concentration. Thus, anything that promotes increasing the micellar size or aggregation number will promote solubilization. These factors include increasing the hydrophobic chain length or decreasing molecular branching. Surfactants with low CMCs tend to be better solubilizers. This is one reason that nonionic surfactants have been used for solubilization in remediation projects. Solubilization is evaluated by phase behavior tests or maximum additive concentration (MAC) tests.
Phase behavior tests involve blending the surfactant solution with the NAPL and allowing it to equilibrate at the temperature of interest. The number of resulting phases and the volume changes are characteristics of the phase behavior (that is, the interaction of the phases).
MAC tests are performed by adding the NAPL in question to prepared surfactant solutions. The NAPL concentration in the aqueous phase can be measured by gas chromatography. All surfactant solutions can be tested at the same surfactant concentration to compare the efficiency of different types. This efficiency can be expressed as a micelle - water partition coefficient. By preparing surfactant solutions of increasingly higher concentrations, the molar solubilization ratio (MSR) for a given surfactant can be determined.
Component solution stability should be monitored throughout the laboratory studies (e.g., the phase behavior should be monitored over time). Solutions that are unstable over time also are unpredictable because their rheological and chemical properties could be changing. If chemical combinations or phases separate during the laboratory testing, that combination can be eliminated from further study. Precipitation of surfactant or other components from solution when mixed with NAPL, groundwater, or matrix material is a cause for concern and alternate surfactant blends should be considered.
Batch Sorption Tests
Surfactant consumption data (i.e., the amount of chemical lost in the subsurface) are needed prior to final selection of a surfactant system. These losses must be made up to maintain the effectiveness of the surfactant solution. Chemical loss data also are necessary for numerical modeling. Batch sorption tests are simple, preliminary tests to estimate the amount of chemical that may be adsorbed to the soil surface or lost by other means. These tests generally combine solutions under consideration with soil from the site. The amount of chemical remaining in solution is measured over time. Samples are taken periodically and the chemical loss is determined by measuring the mass difference between the amount of chemical originally added and the amount still in the solution (less any represented by samples taken). For example, Table 5-17 shows static chemical consumption data after 18 days of solution contact with soil for various surfactant formulations.
|
Table 5-17
|
|
Example Static Chemical Consumption Test Results
|
|
Example Chemical Combination Test Results
|
Consumption
(lb/ yd3) |
|
|
0.5% sodium octyl sulfonate
|
1.62 |
|
0.5% sodium octyl sulfonate (with xanthan gum)
|
1.01 |
|
0.5% sodium octyl sulfonate (with xanthan gum and Na2CO3)
|
2.24 |
|
1.0% ALEO12-157*
|
19.6 |
|
1.0% ALEO12-157 (with xanthan gum)
|
15.7 |
|
1.0% ALEO12-157 (with xanthan gum and Na2CO3)
|
15.7 |
|
0.05% xanthan gum (with sodium octyl sulfonate)
|
0.19 |
|
0.05% xanthan gum (with ALOH12-157)
|
0.19 |
|
|
*ALEO12-15 = linear ethoxylated alcohol, 12-15 carbon chain with 4 moles of ethoxylate
|
Note that the ALEO consumption in Table 5-17 is about an order of magnitude greater than the anionic sodium octyl sulfonate consumption.
Anionic surfactants are not as readily adsorbed to the generally negative net charge on the soil surface compared to nonionic or cationic surfactants. Table 5-18 lists some of the analytical methods used for chemical concentration determinations.
|
Table 5-18
|
|
Chemical Loss to Soil Analytical Methods
|
|
|
Chemical
|
Method
|
Reference |
|
|
Anionic surfactant
|
Surfactant electrode
|
Selig and Fresenius, 1980 |
|
Anionic surfactant
|
Epton titration
|
Epton, 1948 |
|
Nonionic surfactant
|
Spectrophotometric
|
Toel, et al., 1982 |
|
Xanthan gum
|
Total carbohydrate (Flocon 4800)
|
Dubois, et al., 1956 |
|
Polyacrylamide
|
Starch-triiodide
|
Scoggins and Miller, 1979 |
|
Alkalinity
|
Acid titration (APHA #403)
|
Rand, 1975 |
|
Polymer Evaluations for Mobility Control
As discussed in Section 4.3, mobility control may be necessary to provide more efficient sweep when displacing or remobilizing a NAPL with a surfactant solution. Without mobility control, a high-mobility fluid like water will move at a greater velocity than a displaced fluid such as a viscous NAPL. The water will create aqueous channels that provide a better conduit for aqueous solution flow. Once channels have been established between an injection and recovery well, these will provide primary flow paths. This is referred to as viscous fingering, and it is not an efficient displacement mechanism. A much more desirable scenario is the displacement of the NAPL in front of the displacing phase in a more piston-like fashion. This will occur only if the mobility of the fluids is similar, which is one reason to use polymers.
A reasonable laboratory approach is to measure the mobility of a polymer solution in the porous medium. This can be performed using resistance factor data (the relative pressure required to maintain the same flow in a soil column). The resistance factors can be used to determine what concentration of polymer is needed to provide a unit mobility ratio at any fluid advance rate. Modeling the polymer solution as a power law fluid, a log-log plot should show a linear relationship between resistance factor and frontal advance rate for different polymer concentrations. Using this resistance factor data compiled from a linear column experiment, the concentration of polymer required to efficiently displace NAPL can be determined. Additional information on the use of polymers can be found in Sorbie (1990) and Chang (1978).
5.3.5 Laboratory Column and Sand-Pack Tests
Assessments of surfactant/cosolvent performance and the characterization of matrix-fluid interactions are typically performed in soil flushing experiments. These experiments allow refinement of the surfactant systems and determine the NAPL removal effectiveness of selected surfactant systems in actual soil. These tests provide a physical simulation of the soil flushing processes. They are limited by the ability to recreate field conditions in the laboratory, but are nevertheless an essential prerequisite to field pilot testing.
Soil flushing experiments can take many forms. The type selected will depend on the equipment available and the information desired from the results. One-dimensional column tests employ a glass or stainless steel column packed with soil obtained from the site. NAPL from the site can be injected into the column to provide an initial condition. Various solutions then can be flushed through the column to evaluate processes such as surfactantcosolvent sorption, rate-limited mass transfer, cation exchange, polymer effects, and degree of NAPL removal. The permeability of the aquifer material can be evaluated, as well as relative permeability effects if NAPL saturations change throughout the course of the experiment. Depending on the variables and processes being investigated, the column may need to be instrumented to allow measurement of injection and withdrawal flow rates, pressure drop across the column, influent and effluent concentrations, and saturation changes within the column. Ca
re should be taken when evaluating NAPL mobilization in a one-dimensional column, however, because the orientation of the column and the orientation of the flow field can significantly influence the results.
Two-dimensional and three-dimensional sand-pack experiments can be performed to examine the processes discussed above, with the added benefit of better evaluating the influences of heterogeneity, vertical NAPL mobilization, and hydrodynamic instabilities on flood performance. Because each of these processes can have a dramatic impact on the success or failure of a surfactant/cosolvent flood, two-dimensional laboratory experiments should be performed at a minimum. While the cost of these experiments may appear high at first glance, the cost will generally be much lower than that of a failed field pilot test. If a field pilot test fails, there will likely be little motivation to continue with full field-scale implementation.
The exact procedures for conducting column tests and sand-pack experiments could fill many volumes of text, and will not be dealt with further here. It is recommended that a University laboratory or a specialty consulting firm be retained to conduct the required tests. To illustrate some typical test results, Figure 5-14 shows the impact of various injected fluids on the resistance factor in a one-dimensional test. The resistance factor is defined here as:
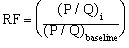 (12)
(12)
where RF is the resistance factor, P is the pressure drop across the column or sand pack at any time (i), and Q is the flow rate of the fluid in question through the column. The baseline ratio of P/Q refers to the end of groundwater injection. It is clear that variations in chemicals change the driving force required to maintain a certain flow rate. Note that during chemical injection the resistance factor increased by a factor of about eight, which would result in the same factor decrease in injectivity. This factor is about four times what was expected due to the increase in viscosity of the injected chemicals. During this experiment, mineral fines were produced, suggesting that something was dispersing clays or silt. Based on the linear coreflood resistance factor results, a simple test was performed to determine what chemical(s) tended to cause dispersion. It was found that the xanthan gum polymer caused the dispersion. The xanthan gum was added to the chemical solution to redu
ce its mobility to a rate more equal to that of the jet fuel so that the surfactant solution could effectively displace and mobilize the fuel. A polyacrylamide polymer can be substituted for the xanthan gum, eliminating the dispersion.
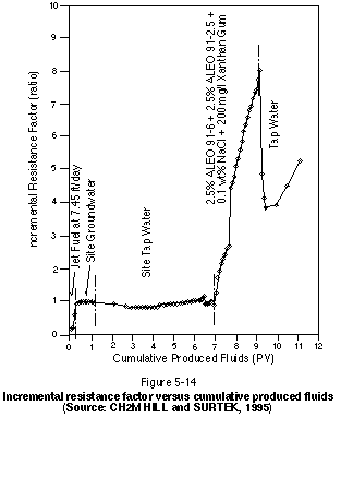
The fluid properties of the solutions produced from a soil flushing experiment also can be evaluated. Cation exchange, solution rheology, and interfacial tension can be monitored. Table 5-19, for example, provides examples of these type of data from a linear coreflood. Note that the injected solution had low interfacial tension, but the interfacial tensions of the produced chemicals were at least an order of magnitude greater. In this example, the loss of interfacial activity was due to the calcium cation exchange. Note that the calcium concentrations in the produced fluids increased to 220 mg/L even though the calcium concentration of the injected solution was only about 30 mg/L.
|
Table 5-19
|
|
Incremental Effluent Chemical Concentrations and Fluid Properties in a Linear Coreflood
|
|
Fluid Injected
|
Cumulative Effluent
(PV)
|
Aqueous Volume (ml)
|
Sodium Octyl Sulfonate (wt%)
|
Effluent Concentration Polyacrylamide (mg/L)
|
IFT (dyne/cm)
|
Ca (mg/L) |
|
|
Injected Solution
|
--
|
--
|
1.000
|
100
|
0.006
|
-- |
|
|
|
|
|
|
|
|
Tap Water
|
1.75
|
33.8
|
0.000
|
0
|
33.6
|
31.0 |
|
|
|
|
|
|
|
|
1.0 wt% sodium octyl sulfonate
|
2.06
|
6.8
|
0.000
|
0
|
>0.7
|
-- |
|
+100 mg/L polyacrylamide
|
2.39
|
6.86
|
0.153
|
0
|
>0.7
|
92.7 |
|
2.71
|
6.91
|
0.535
|
0
|
>0.7
|
-- |
|
3.04
|
6.98
|
0.770
|
0
|
>0.7
|
220 |
|
3.38
|
7.10
|
0.901
|
27
|
0.167
|
-- |
|
3.72
|
7.20
|
0.929
|
45
|
0.126
|
155 |
|
4.06
|
7.22
|
0.962
|
54
|
0.093
|
-- |
|
4.40
|
7.36
|
0.977
|
65
|
0.105
|
182 |
|
4.59
|
3.97
|
0.950
|
68
|
0.081
|
-- |
|
|
|
|
|
|
|
|
Tap Water
|
4.96
|
7.84
|
0.926
|
65
|
0.081
|
77.8 |
|
5.30
|
7.49
|
0.567
|
21
|
0.119
|
-- |
|
5.64
|
7.30
|
0.363
|
3
|
0.126
|
39.4 |
|
5.98
|
7.30
|
0.217
|
1
|
0.142
|
-- |
|
6.32
|
7.30
|
0.151
|
0
|
0.134
|
16.9 |
|
6.66
|
7.30
|
0.114
|
0
|
0.142
|
-- |
|
7.00
|
7.20
|
0.089
|
0
|
0.142
|
8.1 |
|
7.36
|
7.80
|
0.061
|
0
|
0.142
|
-- |
|
7.70
|
7.30
|
0.041
|
0
|
0.134
|
4.9 |
|
8.04
|
7.30
|
0.032
|
0
|
0.099
|
-- |
|
8.41
|
8.00
|
0.023
|
0
|
0.105
|
3.6 |
|
Amount of Chemical Retained by Sandpack
|
|
|
lb/yd3
|
mg/100 gr
|
% *
|
|
|
|
Polyacrylamide
|
0.11
|
3.82
|
61.9
|
|
|
sodium octyl sulfonate
|
0.12
|
3.89
|
0.6
|
|
|
Sandpack dry weight
|
99.88 g
|
|
|
|
|
|
*percent of injected chemical retained by sandpack
|
Besides using surfactant screening studies to identify solutions that may technically recover NAPLs from soil, soil flushing experiments can identify potential problems and solutions to these problems before they are encountered in a pilot test. Changing the operational sequence (that is, the volume and sequencing of various fluids injected) can resolve many potential problems. Figure 5-15 shows the recovery of jet fuel from a radial sandpack experiment.
The operational sequence used in this example involved many of the factors discussed above. Flushing with about 3.5 pore volumes of water recovered about 60 percent of the initial jet fuel saturation in the soil. Because surfactant was optimized with sodium chloride, cation exchange occurred that was demonstrated to be detrimental to the interfacial tension. The calcium was sequestered with sodium citrate to overcome this problem. The first injected solution induced the cation exchange, sequestered the calcium, prevented dispersion with the polyacrylamide, and reduced the solution mobility, also with the polyacrylamide. The second injected solution contained all the same ingredients in an optimized low-interfacial-tension surfactant solution. The resulting mobilization recovered an additional 27 percent of the initial jet fuel saturation for a total of 87 percent, all as free-phase oil. The third surfactant solution injected was a good solubilizer (and provided low interfacial tens
ion), and another 10 percent of the initial saturation was recovered for a total recovery of 97 percent of the initial jet fuel.
There is no one best approach to performing laboratory studies. Each site provides specific constraints and conditions that will limit the direction and scope of the study. The laboratory program itself will evolve as new information is acquired that may raise additional questions. These laboratory studies are time consuming and expensive, but represent an essential portion of the surfactant-based soil flushing design. The preceding sections are not intended as a design process guide, but should provide some indication of the complexity of the undertaking. Table 5-20 summarizes the possible laboratory testing that could be performed.
|
Table 5-20 |
|
Summary of Surfactant/Cosolvent Laboratory Testing
|
| |
Phase |
Test |
Importance |
|---|
| |
NAPL Characterization |
Solubility |
High |
|
Density |
High |
|
Viscosity |
High |
|
IFT |
High |
|
Groundwater and Injected Water
|
Ion Composition |
High |
|
Contaminant concentrations |
Medium |
|
pH |
High |
|
Soil Testing
|
Grain Size Distribution |
High |
|
Cation Exchange Capacity |
High |
|
Batch Sorption Tests |
Medium |
|
Permeability |
High |
|
Contaminant concentrations |
High |
|
Dry bulk density |
Medium |
|
Displacement Pressure |
High |
|
Relative Permeability |
Low |
|
Surfactant Screening |
"Paper" Evaluation
| High |
|
(Impacts of cation exchange, dilution, and salinity on the above)
|
IFT Reduction
Phase Behavior
MAC Tests |
High
Medium
High |
|
Soil Flushing Tests
(Evaluate NAPL removal, chemical losses, produced fluids analysis, permeability impacts, optimization of chemical systems)
|
Column Experiments
Two-Dimensional Sandpacks |
High
Medium |
|
|
|
|
|
|
|
5.3.6 Biodegradability Testing
The biodegradability of a surfactant or cosolvent is valuable in assessing the following conditions:
• The loss of surfactant/cosolvent to biodegradation in the subsurface during injection.
• The fate of surfactants that may be discharged to a wastewater treatment plant (WWTP).
• The fate of in-situ residual surfactant at the completion of a flushing project and the production of potentially harmful byproducts.
During initial screening of surfactants/cosolvents, literature from the manufacturer is often the only readily available information regarding the biodegradation of the purchased chemicals. The biodegradability testing performed by most manufacturers is typically conducted in much different conditions than will exist in the subsurface environment. Consequently, there may be a need to conduct condition-specific biodegradability testing. The need to conduct biodegradability testing will depend on the magnitude of the above concerns. To date, very few tests of surfactant biodegradability have been conducted in conjunction with surfactant flushing projects.
Losses During Flushing
Potential losses during flushing could be evaluated in a flow-through column experiment or a static consumption test. These tests could be similar to those described previously for chemical loss testing. Because biological reactions are typically slower than chemical reactions, the experiments would have to be conducted for a longer period of time. The test can be limited in duration to the expected length of the full-scale surfactant/cosolvent project. Losses that occur after the initial day to week can be assumed to be a result of biodegradation. Secondary indicators of biodegradation, such as oxygen, nitrate, and sulfate consumption, methane, and reduced iron production, could also be monitored to indicate the occurrence of biodegradation.
Fate of Discharge to WWTP
It is possible that a portion or all of the surfactant/cosolvent solution injected into the subsurface will eventually be discharged to a WWTP. The biodegradability testing performed by manufacturers and others typically assumes that surfactants will end up in a biological WWTP because most of these surfactants will get discharged from homes and industries as used wash water. Consequently, the biodegradability testing of the manufacturers is often adequate to demonstrate that biodegradation will occur at a biological treatment-based WWTP. The literature also provides data on the fate of many common surfactants in WWTPs (i.e., Trehy, et. al, 1996). There are data suggesting that the type of treatment used at the WWTP impacts the removal of surfactants and their byproducts (Boethling, 1984). For example, primary treatment is not effective in removing surfactants.
If data are not available from the manufacturer or the published literature, it may be necessary to perform some specific tests. Fairly simple batch tests, or longer term flow-through simulation, could be conducted to evaluate the impact of the surfactants. These tests involve mixing a simulated produced fluid with wastewater at the dilution ratio expected in full-scale operations and feeding this mixture to either a batch system containing a biomass or a flow-through simulator. The impact of the surfactant on biological activity and consumption of the surfactant are then measured over time. Because foaming is a potential adverse impact of the surfactant, the extent of foam production also should be monitored.
Fate of Residual Surfactant
A fraction of the surfactant or cosolvent injected into the subsurface is likely to remain adsorbed to the soil or trapped in pores after completion of the injection phase. Multiple pore volumes of water, or a combination of water and polymer (for mobility control), can be injected following the surfactant to flush out as much residual as possible. The residual surfactant may or may not be a problem in the subsurface. It may serve as a continuous source of dissolved surfactant in the groundwater, which could be problematic if the surfactant has the potential for adverse risks to human health or the environment. It is possible that the surfactant will biodegrade in the subsurface, especially if active bioremediation is used following surfactant injection. Natural or intrinsic biodegradation also may occur. Because oxygen is likely to be a limiting factor at these sites, anaerobic biodegradation may be the primary mechanism for natural bioattenuation.
The biodegradation of the residual surfactant could be measured in column tests or in batch soil systems. Columns should more closely simulate the surfactant trapping that may occur. Water with the appropriate additives could be cycled through columns after they have been flushed with surfactant, or the columns could be kept in a static mode. Because biodegradation may be slow (especially under anaerobic conditions), these tests may take a few months or more to complete. Methods of measuring residual surfactant (or the dissolved surfactant resulting from it) need to be established prior to initiating the test.
5.3.7 Bench-Scale Testing of Produced Fluids Treatment
Handling and treatment of the fluids recovered from surfactant/cosolvent flushing can be a critical component to the success of a surfactant/cosolvent project. Some investigators (Yuill, et. al, 1995) have even concluded that surfactant flushing is not economical unless the surfactant can be recovered from the produced fluids and reused. Although this is a site-specific issue, this view reiterates the necessity of identifying and optimizing effective methods for handling and treating produced fluids. In fact, the ability to cost-effectively handle the produced fluids should be considered and evaluated concurrently with the design of the surfactant/cosolvent solution. Modifications to the solution design may be necessary to develop a system that is effective in contaminant removal and also cost effective.
Figure 5-16 presents a few of the process flow diagrams that have been considered for the handling of produced fluids. Two of the three flow diagrams show reuse of the surfactant solution. The third flow diagram assumes the solution cannot be reused and all of the produced fluids must be discharged. It has been assumed in these diagrams that the extracted fluids will have lower surfactant concentrations than the injected fluids because of the need to overpump the production wells to ensure capture of all the fluids. Consequently, the surfactant solution will need to be reconcentrated, as shown in Figure 5-16, or a bleed stream will need to be treated and fresh surfactant added to the solution. A decision to reconcentrate the solution or add fresh surfactant should be based on the economics of the treatment versus the cost of the surfactant. The unit processes shown in these process flow diagrams are discussed further below.
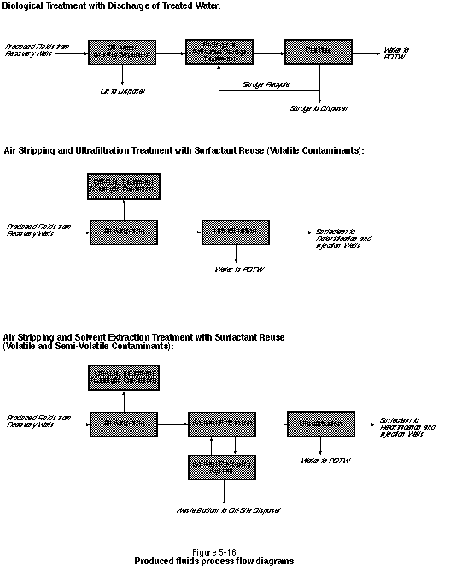
At this point in the development of the surfactant/cosolvent flushing technology, no set methods for handling or treating produced fluids have been established. Because of this, and the fact that the nature of the produced fluids will vary with the surfactant used, the contaminant being removed, and the inorganic makeup of the solution, laboratory testing is necessary to develop an effective produced fluids treatment process.
The possible objectives for the treatment of the produced fluids and some of the processes that have been used and could be used for handling and treatment are discussed below. Each process is described, and a brief procedure is given for running a possible bench-scale test.
Objectives of Treatment
The primary objectives of produced fluids treatment will depend on the specific project scenario and remediation goals. Possible produced fluids treatment objectives include:
• Separation and reuse or recycling of "used" surfactants and cosolvents.
• Separation and reuse of recovered product, if applicable (e.g., fuel oil, solvents).
• Reduction of contaminant levels, oxygen demand, and other pollutants in the produced fluids to the extent necessary and economical before discharging to a municipal WWTP (commonly termed a POTW) or industrial WWTP.
• Production of an effluent that can be discharged to a WWTP without adverse impacts (e.g., foaming).
• Reduction of site contaminants and pollutants in the produced fluids to the extent necessary to discharge the water to a surface water under an NPDES permit.
• Concentration of an organic and/or inorganic phase for separate, off-site disposal or treatment with discharge or reuse of the water.
Unfortunately, treatment of the surfactant/cosolvent flushing solution is sometimes very difficult and costly. The very ability of surfactants to increase the concentration of NAPL in an aqueous stream makes it difficult to later separate the contaminant from the surfactant.
Obtaining Representative Fluids
One challenge in conducting treatability tests of produced fluids is obtaining sufficient quantities of the material to test. It may not be adequate to just mix the surfactant solution with a sample of the NAPL. The chemical and physical characteristics of the fluids are likely to change dramatically as they pass through the soil matrix. Very often the column tests used to design and evaluate surfactant solutions produce only small volumes of fluids. Specially operated columns may be necessary to produce sufficient fluids to test in the laboratory. These columns still may not perfectly simulate the fluids that will be produced in a field system. Consequently, additional testing and evaluation should be performed during a field demonstration of surfactant/cosolvent flushing prior to selection and design of a full-scale system.
Gravity Separation
Gravity separation is the separation of particles from water (or solutions) that are heavier or lighter than the solution. In the case of surfactant/cosolvent flushing, the particles will typically be droplets or globs of NAPL. As coalescence or flocculation occurs, the mass or volume of the particles increases and the rate of separation increases. Oil - water separators can be used that contain coalescing plates that physically assist with coalescence of oil droplets.
Gravity separation is most applicable to surfactant/cosolvent systems that target mobilization as the primary removal process. If the surfactant/cosolvent pushes a front of NAPL out of the formation, this NAPL can hopefully be removed by simple gravity separation.
The end products of a successful gravity separation process could include a free-phase NAPL that can be reused or disposed of separately from the bulk of the water phase. The water phase could possibly be reused in the flushing process, be sent for further treatment, or be discharged directly to a POTW.
A simple settling test can be conducted using a graduated cylinder containing a sample of the produced fluids. The produced fluids are mixed in a cylinder several times, and the flotation rate is determined by recording the position of the float with time. Other testing methods for gravity separation are well established in the wastewater treatment industry (Metcalf and Eddy, 1991).
Dissolved-Air Flotation
In dissolved-air flotation (DAF) systems, air is dissolved in water under a pressure of several atmospheres, and the pressure is then released to the atmospheric level. Water is held in a retention tank under pressure for several minutes to allow time for the air to dissolve. The pressurized flow is then admitted through a pressure-reducing valve to the flotation tank where the air comes out of solution in tiny bubbles throughout the entire volume of liquid. As the bubbles travel upward, they collide with the particles, causing them to rise. A float layer is formed that rises to the surface. If flotation is successful, the float can be skimmed off the surface and the amount of waste will be significantly reduced. The aqueous stream can be sent for further treatment or possibly discharged to a WWTP. Induced Air Flotation (IAF) and froth flotation are processes very similar to DAF, differing primarily in the method of introducing the air bubbles. Excessive foaming is a potential prob
lem with these technologies.
A related process called foam fractionation (MSE Technology Applications, 1996) takes advantage of the foaming because surfactant will tend to concentrate in the foam. The stability of foams is at a maximum at the CMC of the surfactant. Consequently, the concentration process may not be as effective if surfactant concentrations are either significantly less than or greater than the CMC. It is also possible that the contaminants will concentrate with the surfactants in the foam, so that foam fractionation alone may not be an effective process for surfactant/contaminant separation.
Standard procedures exist in the wastewater treatment industry for conducting laboratory testing of DAF. Typical procedures involve pressurizing water in a pressure cylinder of a bench-scale DAF, saturating the water with air by shaking and inverting the pressure cylinder several times, and injecting the air-water solution into a graduated cylinder containing a sample of the produced fluids. The separation and rise rate of float are then observed and quantified.
Chemical Demulsification
Various chemicals can be added to produced fluids in an attempt to reverse the emulsifying effects of the surfactants, allowing the NAPL to phase separate from the produced fluids. Chemical demulsifiers are a group of specialty chemicals that can be added prior to gravity settling or DAF to assist in the separation of NAPL. Chemical demulsifiers are used to treat a wide range of industrial waste oils, spent metalworking and rolling coolants, and other oily waste emulsions. Chemical demulsifiers may alter the chemical composition of the produced fluids such that they are no longer suitable for reuse in the system.
Because most demulsifiers are proprietary, it is often easiest to send samples of the produced fluids to one or more vendors who specialize in this area and have them determine an appropriate demulsifier.
Acid Treatment
Acid treatment also can be used upstream of gravity separation or DAF to assist with NAPL separation. Depending on the surfactant used, acidification may reduce the number of active surfactant molecules through neutralization. Acid also reduces the solubility of the remaining surfactants. This "salting out" effect promotes coalescence by reducing the solubility of the fuel droplets in the emulsion. A potential drawback to acid treatment is the large amount of acid typically needed for phase separation. After neutralization, the salt content of the fluids may be too high for reuse. The high salt content also may make any type of disposal difficult and may require the fluids to be evaporated.
Bench testing acid treatment of produced fluids can be conducted by adding sulfuric acid in varying quantities to obtain pH levels of 1, 2, 3, and 4. Color changes, clarity, oil agglomeration, or floc formation in the solution should be noted. The effect of acid addition on gravity separation also should be noted. Acid treatment at increased temperatures may reduce the amount of acid required.
Heat Treatment
Heat treatment of produced fluids also may aid in NAPL phase separation from produced fluids. Depending on the nature of the surfactant, heat may reduce surfactant solubility. In addition, the solution viscosity should decrease at elevated temperatures, and the effectiveness of droplet collision should increase, thereby promoting coalescence. An advantage of heat treatment is that the surfactant may not necessarily be destroyed at the high temperature, so that after the NAPL is removed and the solution is cooled, part or all of the fluids may be suitable for reuse. Heat treatment can be used in conjunction with chemical treatment.
A water bath using a laboratory hot plate is usually sufficient for this test. The temperatures should be moderate—less than the flash point of the liquids. Color changes, clarity, oil agglomeration, or floc formation should be noted.
Air Stripping
Air stripping is a mass transfer process that relies on the transfer of compounds from the water phase to the gas phase. Air stripping is one of the most commonly used processes for remediation of extracted groundwater contaminated with volatile organic compounds. Air stripping has been evaluated in the laboratory and at the pilot scale for treatment of produced fluids from surfactant flushing projects where volatile contaminants were the targets (Lipe, et. al, 1996; Clarke, et. al, 1992; Fountain, 1992). In this type of application, air stripping offers the potential to remove the volatile contaminants of concern and to allow reuse of the surfactant. Air stripping can be performed by using packed towers, tray towers, spray systems, diffused aeration, or mechanical aeration.
Steam stripping and flash vacuum stripping are modifications to standard air stripping. These processes improve the mass transfer process by operating at a higher temperature or under a vacuum, respectively (MSE Technology Applications, 1996).
The presence of surfactants and cosolvents in produced fluids complicates air stripping. First, significant foaming has been observed in the pilot and laboratory tests that have been performed. Lower flow rates (for both air and water) and anti-foaming agents can possibly be used to control foaming. The presence of the surfactant also hinders the mass transfer of the volatile compounds from the liquid phase to the gas phase. It has been hypothesized that only the contaminants in the dissolved phase will volatilize, and because most of the NAPL is in the micelles, an additional mass transfer step (from inside the micelle to the water phase) is required (Lipe, et al., 1996). Consequently, much larger air strippers would be needed compared to just using water without the surfactant.
The evaluation of air stripping can begin with batch equilibrium experiments. These will provide information on the impact of the surfactant on the Henry’s constant, which can then be used in computer models to predict the size of the systems required. A simple batch, diffused-air stripping test also can be used to asses the potential for air stripping of the fluids. To obtain better design information, pilot-scale air strippers should be used. The volume of produced fluids will be relatively large for such a test.
Membrane Separation
Membranes of various sizes can be used to separate organic and inorganic molecules from water. Ultrafiltration and reverse osmosis are two of the more common membrane processes. The most significant difference between these two processes is the size of the membrane openings. Micellar-enhanced ultrafiltration refers to the increased ability of ultrafiltration membranes to remove surfactants and contaminants from a waste stream due to the colloid-sized surfactant micelle (Lipe, et al., 1996). In a solubilization surfactantcosolvent flushing system, most of the NAPL will be in the micelles; therefore, this type of system may be used to separate the surfactant and contaminants from the water phase. However, because the contaminants will be in the micelles of the surfactant, ultrafiltration may not be useful in separating the surfactant from the NAPL, and the fluid will be unsuitable for reuse. If air stripping is used upstream to remove volatile NAPLs, ultrafiltration may be useful for
reconcentrating the surfactant solution prior to reuse (Lipe, et al., 1996). However, Abdul and Ang (1994) concluded that ultrafiltration would be useful to separate surfactant from a PCB waste stream, thus making it potentially useful for surfactant reuse.
The performance of an ultrafiltration membrane can be characterized by the permeate flux, the percent rejection, and the concentration of solute in the retentate stream (what is held back by the membrane). The permeate flux often decreases with time because of membrane fouling. If the retentate is not suitable for reuse, further treatment (such as evaporation) or disposal of the retentate will be required.
A bench-scale test should be conducted to determine if ultrafiltration will be successful in concentrating the solution. This test can be run with a bench-scale ultrafiltration unit either by the interested party or by a vendor who specializes in this area. While conducting the test, the following should be noted:
• Flux through the membrane (feed solution as well as a pure water stream)
• Temperature and pressure during the test
Permeate and retentate samples should be taken after a sufficient amount of fluid has passed through the membrane.
Biological Treatment
Biological treatment systems utilize microorganisms to degrade organic wastes. Organic substances are mineralized (transformed to carbon dioxide and water), transformed into other organic compounds with a lower biological oxygen demand, and finally transformed into biomass. Because most surfactants and cosolvents are biodegradable, biological treatment should be effective in treating the surfactant/cosolvent in the produced fluids. Some contaminants of concern may not be readily biodegradable, and therefore may not be destroyed. However, volatile compounds could be removed in an aerated treatment system through volatilization.
Biological treatment will result in the destruction of the surfactant or cosolvent, so that it cannot be reused or recycled. Biological treatment may be the only viable alternative for the treatment of surfactant in a bleed stream or the final pore volume of surfactant at the completion of a project. These fluids cannot be reused and must be treated and discharged off site. Biological treatment was used to treat surfactants and oils in field pilot studies by Abdul, et al. (1992) and at the Laramie, Wyoming site (see Appendix A).
Aerobic processes are typically favored or required for treating produced fluids. Fairly standard aerobic wastewater treatment methods can be used, although the produced fluids may have a number of peculiarities that must be taken into account. These include the fluids’ high organic strength (1 percent surfactant solution may result in 10,000 mg/L of chemical oxygen demand), high sodium content, and foaming potential. Raw produced fluids may be inhibitory to microorganisms either because of the surfactant/cosolvent or the contaminants present. Appropriately engineered systems (e.g., long retention times) are required to deal with these factors.
Testing of biological treatment systems typically requires a fairly long time period (for example, 2 months) and, consequently, a large amount of produced fluids. Batch, fill and draw, or continuous-flow type activated sludge laboratory systems could be used. Standard procedures for testing biological wastewater treatment can be found in the literature (e.g., Eckenfelder and Musterman, 1995).
Evaporation
The objective of evaporation is to concentrate a solution consisting of a nonvolatile solute and solids by evaporating the water and volatile compounds. Very simply, the fluids are heated to the boiling point to drive off the water. The vapors coming off the evaporator may need to be treated and/or condensed if volatile contaminants are present. The concentrated liquid remaining after evaporation will typically be drummed and sent off site for disposal.
The most cost-effective evaporators are typically sized to handle a few thousand gallons of fluids per day. Consequently, evaporation may be suitable only for small projects or as an additional step in concentrating brines from membrane-separation processes. Foaming is a potential problem with the evaporation of fluids containing surfactants.
Fairly simple laboratory bench-scale tests can be conducted to determine if evaporation will be successful in concentrating the solution. Vendors specializing in evaporators should conduct tests prior to implementation of a large-scale test.
Solvent Extraction
Solvent extraction involves mixing a solvent with the produced fluids so that the organic contaminants will transfer from the surfactant solution phase to the solvent phase. The solvent must have a favorable partitioning coefficient for this mass transfer to occur. The solvent also must have a density significantly different than the surfactant solution so that it will easily gravity-separate from the solution. Some type of low-energy mixing may be used to provide contact between the solvent and the produced fluids. Solvent extraction has been investigated for the separation of non- and semivolatile organics from produced fluids (Clarke, et. al, 1992). The "waste" solvent from the solvent extraction unit may be recovered in a solvent recovery system (typically a distillation system).
Because solvent extraction relies on mass transfer, it is not possible to achieve complete removal of the contaminants from the surfactant solution. As with air stripping, the presence of the surfactant is likely to reduce the efficiency of the mass transfer compared to pure water. It is also possible that some of the added solvent will end up in the surfactant solution, which may or may not impact the reusability of the surfactant. Despite these difficulties, solvent extraction is one of the few processes that has the potential for separation of a non- or semivolatile contaminant from a surfactant solution, thereby allowing reuse of the surfactant.
Evaluation of solvent extraction can begin with simple laboratory extractions using separatory funnels. After mixing the solvent phase with the produced fluids, the phases can be separated and analyzed separately for the contaminants of concern. Specialty vendors could also be used to evaluate the applicability of proprietary solvent extraction systems.
Pervaporation
Pervaporation is a membrane separation process that is driven by differences in volatility of the feed components and permselectivity (permeation-based selectivity) of the membrane. Traditionally used in the chemical industry to break azeotropic water - alcohol mixtures and to perform separations when distillation is highly energy intensive, pervaporation has received increasing attention in the past decade for application in environmental problems. Successful field demonstrations have shown that 99.9-percent removal of TCE from groundwater recovered in a conventional pump-and-treat system can be achieved using pervaporation. More recently, pervaporation has been investigated for the removal of volatile NAPLs from waste and process streams containing surfactants (MSE Technology Applications, 1996).
In pervaporation, a liquid stream containing VOCs is placed in contact with one side of a non-porous membrane while a vacuum or gas purge is applied to the other side. The volatile components in the liquid stream sorb into the membrane, permeate through the membrane, and evaporate to form a vapor phase (hence the word pervaporate). The vapor phase is then condensed to form a liquid permeate. Thus, volatile NAPLs are separated from the non-volatile surfactants, which can be recovered and reused. By using hydrophobic membranes that are selective for the volatile component being removed, a high separation factor for NAPLs (100-3000) can be achieved. In groundwater applications, the membranes used are typically rubbery polymers such as silicone rubber, polybutadiene, natural rubber, etc. In the presence of surfactants, there is a reduction in the driving force for transfer of the VOC out of the solution due to the equilibrium established between the VOC and surfactant in solution. Ther
efore, there is a decrease in the rate of VOC removal. The degree of reduction depends on the types and concentrations of surfactants and VOCs, as well as the membrane material. This effect can be offset by adding membrane area or increasing the process temperature.
Membrane air stripping is a modification of pervaporation in which porous hollow fibers are used (MSE Technology Applications, 1996).
Pervaporation can be tested in the laboratory on a variety of surfactant and NAPL solutions using laboratory membranes. Manufacturers of membranes can typically assist with construction of the units. Pilot-scale units also can be constructed and tested.
Oxidation
It may be possible to use a number of oxidation technologies to treat produced fluids. Wet air oxidation is one such method. Oxidation of organic compounds occurs under high temperature and pressure in this process. Oxidation with ozone or hydrogen peroxide and catalysis with UV light is also possible. However, the total concentration of the organic in a produced fluid is likely to be so high as to make oxidation with these types of chemicals economically infeasible. If a pretreatment separation step is used, these type of oxidation methods may be practical.
Laboratory methods for evaluating oxidation technologies are available. Vendors of these technologies may be able to perform initial laboratory studies. Vendors may also be able to supply pilot-scale units to evaluate these technologies.
Addition of Absorption Media
Certain vendors have developed clay-based powder chemicals that will remove emulsified oil and metals from water in concentrations as high as 2 percent of fluid volume. A single powdered reactant is added and mixed with the wastewater. The emulsion is then broken and the other contaminants are scavenged and fixated onto large floc particles through a process of flocculation and encapsulation. The result is a sludge cake, which may be suitable for disposal in a landfill. The remaining treated solution may be sufficiently free of oil, metals, and suspended solids to be recycled or discharged to a municipal sewer.
5.4 Numerical Simulation
Relevance
At most sites where surfactant/cosolvent flushing is being considered, a numerical model is an essential tool for system design. Although individual processes can be evaluated in the laboratory, the a-priori performance of the system as a whole often can only be examined through numerical simulation. In general, a numerical model should be used to design the pilot field test, and then calibrated using the results of this test to allow design of the full-scale remediation effort. Readers with experience in the use of numerical simulators for surfactant/cosolvent flushing design may choose to skip this section.
5.4.1 The Modeling Process
The numerical modeling process begins by defining the objectives of the modeling exercise. In most cases, the objectives of the numerical modeling exercise may include answering any of the following questions:
• What will be the rate of contaminant removal as a function of time or pore volumes injected?
• What will be the influence of various processes such as surfactant/cosolvent sorption, degradation, or nonequilibrium mass transfer on system performance?
• What concentration and volume of surfactant or cosolvent needs to be injected to achieve a certain degree of mass removal?
• What are the possible migration paths of contaminants and injected fluids?
• Could attempts at remediation remobilize contaminants into previously uncontaminated regions of the subsurface?
• What effect do heterogeneities have on mass removal?
Once the modeling objectives have been defined, a conceptual model must be formulated. A conceptual model is an idealization of the real system incorporating the relevant processes and the degree of interaction required between these processes. If the site of interest contains low-permeability lenses such as clay or silt, for example, then diffusion-limited mass transfer from these lenses may be a relevant process. Conceptualizing the site as a homogeneous domain may not be appropriate. If the type of surfactant to be injected is known to degrade in aqueous solution, then conceptualization of the surfactant as a conservative species may not be appropriate. A decision also must be made at the conceptual model stage with respect to the dimensionality of the employed model. Although all sites are three-dimensional, 3D models are typically expensive to operate. In general, one should employ the fewest number of dimensions to satisfy the modeling objectives. A proper conceptual model ca
n only be formed once adequate field and laboratory screening data are available.
Following formulation of the conceptual model, a mathematical model must be created. It is at this step that the physical and chemical processes are formulated in terms of a boundary value problem that comprises the governing partial differential equations, relevant constitutive relationships, material properties, boundary conditions, and initial conditions. The partial differential equations governing surfactant/cosolvent systems can be found in a variety of references, including Abriola, et al. (1993) and Brown, et al. (1994).
When utilizing a commercially available mathematical model, the model user must ensure that it incorporates the physical and chemical processes required to satisfy the site conceptual model. If it is determined that viscous instability may be a relevant process in an alcohol flood, for example, then the selected model must be able to account for changes in viscosity with alcohol and contaminant concentration. The model user also must be prepared to support the mathematical model with site-specific constitutive relationships such as capillary pressure curves, relative permeability curves, sorption isotherms, mass transfer coefficients, etc. Although some of the required constitutive relationships can be estimated from the published literature, others will need to be measured as part of a laboratory testing program.
Once the mathematical model has been formulated, the governing partial differential equations need to be solved. For most problems, this requires the use of a numerical solution technique such as the finite difference method, finite element method, or finite volume method. Once the numerical solution technique has been developed, a computational algorithm can be created by transposing the numerical solution to a computer code using a chosen programming language. For a commercially available model, the numerical solution technique and programming language will have been selected by the model’s authors.
In general, the computational effort required to run a surfactant/cosolvent numerical model will depend on the dimensionality of the model and the number of nonlinear processes incorporated. A three-dimensional model that accounts for processes such as NAPL migration and nonequilibrium mass transfer, for example, will typically require a CPU-intensive workstation with a large amount of memory. A simple one-dimensional model assuming equilibrium partitioning, on the other hand, may be run on a personal computer. Numerical simulation of surfactant/cosolvent flushing systems generally requires a level of user effort and expertise far beyond that needed to simulate less complex problems such as groundwater flow and single-component solute transport. If a relatively complex conceptual model has been adopted, the services of an experienced numerical modular should be retained to complete the modeling exercise.
Once a computer code has been created, it must be verified to ensure that the code is free of errors. This task is the responsibility of the model authors. In addition to verification, the mathematical model should be validated to ensure that it correctly represents the physical and chemical processes of the system. Validation is typically carried out by comparing model results to those of a controlled laboratory experiment. For a commercially available model, the model user is advised to determine if verification and validation exercises have been carried out by the authors. If these exercises have not been done, the user should be aware that the selected model may produce unreasonable results.
The performance of a field pilot test or appropriate laboratory tests provide the opportunity for model calibration. Calibration involves fitting the model output to observed data while adjusting certain input parameters. Once the "best-fit" input parameters have been determined, these are deemed to be appropriate for carrying out predictive simulations. Ideally, as few parameters as possible should be varied during the calibration exercise. Whenever parameters are adjusted, the possibility of a non-unique solution must be considered. If this is a serious concern, additional laboratory or field tests must be conducted to constrain the parameters in question. Once the selected numerical model has been calibrated, simulations can be carried out to satisfy the objectives of the modeling exercise.
5.4.2 Review of Published Models
In general, any numerical model that simulates phase migration subject to mass transfer between phases can be used to simulate surfactant/cosolvent flushing of soil and groundwater provided that appropriate constitutive relationships are incorporated. Although numerous multiphase/multicomponent models appear in the published literature, only some of these have been used to actually simulate surfactant/cosolvent systems. This brief review will be limited to a selection from those models only.
At present, the most comprehensive numerical model for application to surfactant/ cosolvent systems appears to be UTCHEM, developed by Brown, et al. (1994). The authors present a three-dimensional finite difference model capable of simulating the transport of up to 19 components in a four-phase system. The model can incorporate a variety of equilibrium phase behavior relationships, allowing it to simulate alcohol flooding, surfactant flooding, and combined surfactant/cosolvent flooding applications. Phase viscosities, densities, and interfacial tensions are calculated as functions of phase composition. Capillary pressure and relative permeability curves are adjusted according to interfacial tension through the capillary number. The model also accounts for surfactant sorption.
Brown, et al. (1994) apply their model to investigate the time and amount of surfactant required to remove relatively large volumes of tetrachloroethylene (PCE) from a heterogeneous, sandy aquifer. The removal mechanism investigated is enhanced solubilization by application of an anionic surfactant mixture. Although the authors state that a nonequilibrium mass transfer approach is available in the model, all simulations presented in the study assume equilibrium partitioning between DNAPL and aqueous surfactant solution.
Abriola, et al. (1993) present a one-dimensional numerical model to simulate surfactant-enhanced solubilization of NAPL in porous media subject to nonequilibrium mass transfer between NAPL and an aqueous surfactant solution. A stationary residual NAPL phase is assumed. The model is calibrated to laboratory experiments involving the solubilization of dodecane, demonstrating very clearly the need for a nonequilibrium mass transfer formulation. Mason and Kueper (1996) present a similar one-dimensional model where the nonequilibrium mass transfer term accounts for high non-wetting phase saturations found in NAPL pools. The developed model is compared to laboratory column experiments involving the solubilization of pooled PCE. Reitsma and Kueper (1996) present a one-dimensional model to simulate the use of alcohol flooding to remove DNAPL from below the watertable. The results of this study demonstrate that slug deterioration due to hydrodynamic dispersion can dramatically influence the
degree of mass removal achieved in an alcohol flood.
In addition to numerical models developed by the contaminant hydrogeology community, a variety of surfactant/cosolvent models exist in the petroleum industry. Numerical simulation of multiphase/multicomponent flow and transport has been routinely performed by the petroleum industry for the past 30 years. UTCHEM, discussed above, originated as a software package to investigate the use of micellar-polymer floods in the oil field (Brown, et al., 1994). Another model, GCOMP (Gao, et al., 1996), has been used to simulate the alkaline-surfactant-polymer process at the Daqing oil field pilot project. GCOMP is a three-dimensional model with a chemical option that accounts for density and viscosity changes, the influence of interfacial tension on capillary number and relative permeability, and chemical partitioning to the soil matrix.
5.5 Field Demonstrations
Relevance
Once a surfactant/cosolvent flushing system has been designed on the basis of laboratory testing and numerical simulation, a pilot field test needs to be carried out. The pilot test serves as a small-scale field demonstration of the remediation effort. The pilot test is an essential step in proceeding to full-scale implementation and should not be eliminated. This section provides information on the implementation of field demonstrations. Much of this information also is applicable to full-scale implementation. Readers interested in implementing a surfactant/cosolvent flushing project should read this section.
Key Points
• Field demonstrations are typically needed for surfactant/cosolvent flushing applications because the technology is relatively new, and because the risks in terms of cost and environmental damage are relatively high. It is also difficult to accurately scale up from the laboratory to field applications the performance and potential problems of these systems, so that a field demonstration are prudent prior to investing in a full-scale application. The feasibility of surfactant/cosolvent flushing should be re-evaluated after field demonstrations have been performed.
• Objectives of field demonstrations typically include evaluation/demonstration of contaminant removal performance, refinement of chemical delivery sequence, evaluation of design parameters, testing of equipment, testing of produced fluids treatment methods, and refinement of costs.
• Work plans are typical necessary prior to implementation of field demonstrations.
• A variety of permits and regulatory approvals may need to be obtained prior to implementation of the field demonstrations.
• Key design issues for field demonstrations include selection of scale, the use of containments, the recovery/delivery system to use, selection of location, the chemical delivery sequence to use, and the chemical supply equipment to use. Factors to be considered and examples at previous field demonstrations for these issues are presented in this section.
• One primary method for handling the produced fluids must be established for a field demonstration.
• Further pilot testing of produced fluids handling methods can be performed during the field demonstration.
• Factors to consider during the construction, operations, monitoring, and reporting of a field demonstration are presented in this section.
• Field demonstrations provide a better indication of the performance of a full-scale system, but they have their limitations so that there is no guarantee that they will perfectly predict the performance of a full-scale system.
5.5.1 Need for Field Demonstration
With any new technology, it is typically not prudent to move from a laboratory study to full-scale implementation without some type of field demonstration or pilot test. Because the risks, in terms of cost and environmental damage, of surfactant/cosolvent flushing systems are relatively high, field demonstrations are essential. Before injecting large amounts of surfactants or cosolvents into the subsurface, it is prudent to inject a relatively small amount and verify that there is no undesired migration of contaminants or chemicals. The disposition and handling of the produced fluids also is best evaluated at a small scale before larger quantities of the fluids are produced.
Additionally, field demonstrations are warranted because of the difficulty in scaling up from laboratory tests to field application. It is very difficult, if not impossible, to accurately simulate in the lab the heterogeneities that are present in the field. Furthermore, even the best laboratory work cannot predict all the potential issues that may arise at the field scale. Numeric simulations can improve on the predicted performance of a full-scale system, but there are currently no numeric simulations that can model all of the chemical and physical heterogeneities present in natural subsurface regimes. Consequently, it is impossible to predict with sufficient certainty the performance of a surfactant/cosolvent system without performing a field demonstration.
5.5.2 Objectives of A Field Demonstration
The objectives of a field demonstration could include:
• Demonstration and evaluation of contaminant removal performance. This should be in terms of the overall objectives of the remediation, as discussed in Section 5.1.
• Refinement of the surfactant/cosolvent system and delivery sequence. The specific chemicals to be used for the flushing system will, for the most part, be selected in the laboratory studies. However, refinements will probably be required based on the actual field performance.
• Evaluation of design parameters, such as injection flow rate, needed for full-scale design.
• Testing of equipment and processes for making up, injecting, and recovering the chemical solutions.
• Refinement of the cost estimate. Based on the additional information gained during the field demonstration, a much more realistic cost estimate for the full-scale system can be prepared.
5.5.3 Work Plans
One of the first tasks in the performance of a field demonstration is the preparation of a work plan and other associated documents, including a Sampling and Analysis Plan, a Quality Assurance Project Plan, and a Health and Safety Plan. Suggested formats for these documents are provided by the EPA (USEPA, 1989). The specific format requirements typically will be dictated or negotiated with the client and the regulatory officials.
5.5.4 Permits and Regulatory Approval
A key component in performing a field demonstration is obtaining the necessary regulatory approval. This approval may or may not include specific permits, depending on the regulatory status of the site.
Regulatory Approval
Even if they have no specific permitting authority, the regulators of the site (federal, state, and possibly local/regional) typically need to accept the concepts and objectives of the field demonstration. To facilitate this acceptance, it is often prudent to include the regulators as part of the team that originally selects surfactant/cosolvent flushing as a remedy and to involve them in the development of the process. If specific permits are not required, review and approval of the work plans are typically the formal mechanism by which regulatory approval is obtained. For the Department of Defense, Department of Energy, and other federal agencies, an Interagency Agreement may spell out the regulatory approval process required.
Two EPA publications, "Surfactant Injection for Ground-Water Remediation: State Regulators' Perspectives and Experiences" (USEPA, 1995a) and "State Policies Concerning the Use of Injectants for In-Situ Ground-Water Remediation" (USEPA, 1996), provide excellent reviews of the regulatory requirements for obtaining state approval. These documents should be consulted prior to undertaking a field demonstration. For example, USEPA (1995a) summarizes the concerns of states identified in a survey as follows:
The survey identified state concerns about the toxicity of the surfactant, masking effects (on analytical tests), transfer of contaminants from soil to ground water, satisfactory hydrologic control, and adequate monitoring to ensure that processes taking place in the subsurface are understood. In particular, state regulators need to be convinced that use of surfactants will not make the situation worse, that NAPLs are not mobilized without being recovered, and that the surfactant itself can be recovered or remediated.
Underground Injection Control (UIC) regulations may require a permit for surfactant/ cosolvent injection in some locations. The survey conducted by EPA (USEPA, 1995) found that UIC permits were typically not required for pilot studies. UIC permits may, however, be required for full-scale systems. In addition, some state water quality standards prohibit the use of surfactants that contain Safe Drinking Water Act (SDWA) constituents at concentrations above the maximum concentration level (MCL).
Acceptance by Site Owner
Researchers sometimes perform field demonstrations on a site, with little or no financial backing by the site owner. The site owner is likely to be very concerned about potential damage to his site (e.g., making the problem worse by mobilizing contaminants) and is therefore likely to scrutinize the field demonstration very closely. The site owner may hire an outside consultant to assist with the review of the demonstration work plan. As with the regulators, site owners need to be brought into the decision-making process early so that they can provide input into the design of the demonstration. This should help facilitate their approval of the design.
Produced Fluids and Residual Disposal
Inevitably, field demonstration will result in recovered and produced fluids that will need treatment and/or disposal. The disposal of these fluids and the associated permits needs to be considered in the planning stage of a field demonstration.
If the volume of the fluids is relatively low, it may be cost-effective to haul all of the fluids off site for treatment/disposal. Incineration of the fluids may be required. If it is possible to separate somewhat "pure" NAPL, it may be possible to recycle this material at a fuel blending facility. Typically, the waste hauler and incinerator facility will be familiar with the regulatory and paperwork requirements for shipping and disposing of the fluids. The generator of the fluids should review the procedures to ensure that all applicable regulatory requirements are being followed.
Discharge to a WWTP may be a viable alternative, although some pretreatment may be required. A pretreatment permit will typically be required from the agency owning the WWTP. Discharge to a surface water body is another alternative. This will require an NPDES permit, typically from the State. There are standard procedures for obtaining NPDES permits, which typically takes about 6 months.
Air Permits
Depending on the nature of the contaminants and the method used to treat the produced fluids, it may also be necessary to obtain an air discharge permit. For example, with a chlorinated solvent waste, air stripping could be used to separate the contaminant from the surfactant. It may be possible to directly discharge the off-gas from the air stripper for a field demonstration if the total mass emitted is low, the duration is short, and the rate of emissions is low. An air discharge permit may be required if the mass emitted is greater than some threshold, typically dictated by the State. Modeling also may be required to demonstrate that the air quality at a potential receptor site is not above allowable standards.
Air emissions could be a concern for fuel hydrocarbon contamination with biological treatment or similar technologies that result in aeration of the produced fluids. Permits and/or off-gas control may be required for these types of facilities.
5.5.5 Selection of Scale
Selecting a scale for a field demonstration depends on site characteristics and project-specific objectives. The following are key considerations in selecting a scale for field demonstrations.
Representative of Subsurface Conditions
The target zone should to be large enough to be representative of site conditions such as heterogenities and NAPL distribution.
Space for Soils Sampling
Sufficient space needs to be available to collect a representative number of soil samples before and after fluid cycling to demonstrate effectiveness. Space requirements may be defined by sampling equipment (e.g., hollow stem auger rigs) and sampling patterns.
Constructable
Sufficient space needs to be provided to construct the required systems. For example, pile drivers for sheet pile walls, backhoes for trench construction, and tankage for fluids all have significant space requirements.
Mechanically Representative of Full-Scale
In addition to testing in-situ effectiveness, field demonstrations can help to resolve and optimize mechanical design issues such as types of pumps, material compatibility, instrumentation and controls, physical containment systems (e.g., sheet pile walls), fluid delivery/recovery systems, and produced fluids treatment systems. It may be desirable for the field demonstration to be large enough to resolve mechanical issues so that better cost estimates for full-scale applications can be developed.
Reasonable Time and Costs
The target needs to be small enough that the field demonstration can be completed in a reasonable period of time and at an acceptable cost. The costs of the field demonstration need to be put in perspective to the potential cost for any full-scale surfactant/cosolvent system.
Scales of historical field demonstrations are listed in Table 5-21.
|
Table 5-21
|
|
Scale of Field Demonstrations
|
|
|
Project
|
Size
|
Target Depth |
|
|
Hill AFB, Utah - OU1 Cell #3
|
3 by 5 m
|
5 to 9 m bgs |
|
Laramie, Wyoming-Large
|
40 by 40 m
|
2 to 2.5 m bgs |
|
Canadian Forces Base Borden, Ontario
|
3 by 3 m
|
1 to 3.4 m bgs |
|
Fredricksburg, Virginia
|
Not Available at This Time
|
Not Available at This Time |
|
Hialeah County, Florida
|
3 by 3 m
|
Approximately 1.2 m bgs |
|
Corpus Christi, Texas
|
7.6 by 11.7 m
|
3.6 to 7.3 m bgs |
|
Warren, Michigan
|
3 m Diameter
|
0 to 1.5 m bgs |
|
Quebec City, Quebec
|
Not Known at this Time
|
1.8 to 4.3 m bgs |
|
Hill AFB, Utah - Ethanol
|
3 by 5 m
|
4.6 to 6.1 bgs |
|
Hill AFB, Utah - Surfactant
|
3 by 5 m
|
5 m bgs |
|
Laramie, Wyoming - Small
|
5 by 5 m
|
2.6 to 3.2 m bgs |
|
Hill AFB, Utah - Complexing
|
3 by 5 m
|
?? to 3 bgs |
|
Fort Worth, Texas
|
Not Known at This Time
|
Not Known at This Time |
|
Hill AFB, Utah - Surfactant
|
3 by 5 m
|
4.9 to 7.9 m bgs |
|
Hill AFB, Utah - OU2
|
6 by 9 m (1PV=15,000
|
8 to 15 m bgs |
|
Hill AFB, Utah - OU2 Foam
|
Not Known at This Time
|
Not Known at This Time |
|
Traverse City, Michigan
|
3 in Diameter
|
15 to ?? m bgs |
|
L'Assomption, Quebec
|
4.3 by 4.3 m
|
1 to 2 m bgs |
|
Paducah, Kentucky
|
Approximate 2 m Radius
|
20 to 30 m bgs |
|
Delmont Station, Pennsylvania
|
Fracture Approximately 150
|
3 to 9 m bgs |
|
Picatinny Arsenal, New Jersey
|
18 by 6 m
|
3 to 15 m bgs |
|
Piketon, Ohio
|
5 by 2 m (1PV=3700 gal)
|
9,9-10.4 m bgs |
|
Commercial Site, New Jersey
|
Not Known at This Time
|
Not Known at This Time |
|
Hill AFB, Utah - Surfactant
|
3 by 5 m
|
?? - 5 m bgs |
|
|
|
|
5.5.6 Containment and Recovery/Delivery Systems
Many of the field demonstrations conducted to date have been contained within a sheet pile wall barrier. For example, nine of the 26 demonstrations described in Appendix A include sheet pile barrier walls. In the remaining 17 cases, hydraulic systems have been used to contain the chemicals. The general objective of containment could be to reduce the short-term risk of exposure during the remediation. More specific objectives of containment could be to:
• Prevent NAPLs outside of the cell from moving into the cell. This would potentially bias mass balance calculations.
• Contain the elevated contaminant concentrations resulting from the solubilizing effect of the surfactant/cosolvent.
• Contain delivered chemicals within a known area to mitigate potential adverse impacts.
• Provide a physical barrier for control of subsurface flow patterns.
• Minimize production of clean water from outside the cell that might complicate treatment of produced fluids and/or reuse of components of the delivered chemical system.
With regard to subsurface delivery/recovery systems, a variety of approaches can be used. Examples from Appendix A are listed in Table 5-22.
|
Table 5-22
|
|
Delivery/Recovery Systems Used
|
|
|
Project
|
Delivery/Recovery Systems |
|
|
Hill AFB, Utah - OU1 Cell #3
|
Line Drive - 4 Delivery Wells and 3 Recovery Wells (PVC) |
|
Laramie, Wyoming-Large Field Demonstration
|
Horizontal Drainlines (Polyethylene Pipe) |
|
Canadian Forces Base Borden, Ontario
|
Line Drive Delivery and Recovery Wells (5-cm PVC) |
|
Fredricksburg, Virginia
|
Wells |
|
Hialeah County Florida
|
A Single Horizontal Delivery Drain, 4 Vertical Recovery Wells |
|
Corpus Christi, Texas
|
Line Drive Delivery and Recovery Wells |
|
Warren, Michigan
|
Vertical Infiltration With a Central Extraction Well (PVC) |
|
Quebec City, Quebec
|
400 Delivery/Recovery Points Arranged in Three Networks |
|
Hill AFB, Utah - OU1 Ethanol
|
Line Drive - 4 Delivery Wells and 3 Recovery Wells (PVC) |
|
Hill AFB, Utah - Surfactant Solubilization, OU1, Cell #5
|
Line Drive - 4 Delivery Wells and 3 Recovery Wells (PVC) |
|
Laramie, Wyoming - Small Field Demonstration
|
Horizontal Drainlines (Polyethylene Pipe) |
|
Hill AFB, Utah - Cyclodextrin Solubilization, OU1 Cell #4
|
Line Drive - 4 Delivery Wells and 3 Recovery Wells (PVC) |
|
Fort Worth, Texas
|
Tentatively Line Drive |
|
Hill AFB, Utah - Surfactant with Cosolvent OU1 Cell #8
|
Line Drive - 4 Delivery Wells and 3 Recovery Wells (PVC) |
|
Hill AFB, Utah - OU2 Micellar Flood
|
Line Drive - 3 Delivery Wells and 3 Recovery Wells (PVC) |
|
Hill AFB, Utah - OU2 Foam
|
Line Drive Between Three Delivery and Three Recovery Wells Spaced 30 feet Apart |
|
Traverse City, Michigan
|
Vertical Circulation Well (Stainless Steel) |
|
L'Assomption, Quebec
|
Wells (Five Spot Pattern with Central Delivery) |
|
Paducah, Kentucky
|
Single Well For Delivery and Recovery |
|
Delmont Station, Pennsylvania
|
One Delivery Well, Recovery at a Spring |
|
Picatinny Arsenal, New Jersey
|
Wells |
|
Piketon, Ohio
|
Single Recovery Well, Single Delivery Well |
|
Commercial Site, New Jersey
|
Wells |
|
Hill AFB, Utah - Surfactant Mobilization, OU1 Cell #6
|
Line Drive - 4 Delivery Wells and 3 Recovery Wells (PVC) |
|
|
|
Selection of a delivery/recovery approach is driven by optimization of sweep efficiency, site features (primarily depth), equipment availability, and cost considerations. Typically, the best approach is found through analytical and/or numerical modeling of fluid flow patterns. Modeling methods are described in detail in Section 5.4.
5.5.7 Selection of Location
Selecting a location for a field demonstration is a critical step. At a basic level, minimum requirements for a field demonstration include the following:
• The NAPL of concern must be present at levels representative of the majority of the site. It may be a difficult task to find such a location.
• Soils must be sufficiently permeable to cycle fluids.
• The consequences of reasonable worst-case scenarios (e.g., formation plugging) must be acceptable.
• Physical access should not require significant investment.
• Access to utilities (e.g., water, power, and phone) should not require major investment.
• Field activities need to be compatible with ongoing land use.
Given the above conditions, a determination needs to be made as to whether the demonstration should be conducted at the most favorable location, a representative location, or a reasonable worst-case location. Pros and cons of each of these approaches are presented below.
Favorable Locations
Implementing a field demonstration involves numerous technical and design challenges. Working at a favorable location greatly increases the overall chances for a successful field program. The downside of this approach is that in ignoring more difficult locations, the limiting case at a given site may not be properly defined.
Representative Locations
Backing off from an ideal location to one that is more typical has the advantage of yielding a more reasonable evaluation of effectiveness, costs, and benefits. On the other hand, taking "too large a step" from laboratory to field implementation may cause the project to fail for reasons that might have otherwise been avoided (e.g., formation plugging).
Reasonable Worst-Case Locations
Overall, what can be achieved at a site will be defined by the worst-case location. For example, if surfactant/cosolvent flushing is only effective at half a site, it is unlikely that the overall benefits (reductions in risk or site care requirements) will justify the costs. This line of logic suggests that worst-case locations need to be considered.
5.5.8 Chemical Delivery Sequence and Schedule
A critical element for the design of the field demonstration is the chemical delivery sequence and schedule. From this schedule, the chemical supply equipment, delivery and recovery pumping systems, and produced fluids handling systems can be designed.
The first element in this effort will be an estimate of the number of pore volumes of chemicals required and the volume of a pore volume for the specific well configuration. As discussed previously, numerical simulations can be used to aid this effort. Simple volume estimates also can be used.
Summaries of fluid delivery sequences are included in the summary tables in Appendix A.
5.5.9 Chemical Supply Equipment
The complexity of the equipment needed to prepare and deliver the surfactant or cosolvent systems will depend on the nature of the required chemical solutions and the scale of the system. For small-scale systems requiring only a few thousand gallons of chemicals, it may be cost-effective to have the chemical supply company "pre-make" the solution. Care will have to be taken with such solutions at the site because they are likely to have a limited shelf life.
For larger systems requiring larger volumes of chemicals, it may be more cost-effective to have concentrated chemicals delivered to the site and mix the solutions on site. It may be necessary to first soften the dilution water, depending on the chemical composition of the local potable water or groundwater and the sensitivity of the surfactant solution to calcium and other multivalent cations. The concentrated surfactants, cosolvents, or polymer will typically be delivered as liquids. For chemicals that are easily biodegraded, such as xanthum gum polymers, the tanks and piping used for their storage must be kept clean and the time of storage minimized. These liquid chemicals can be diluted and mixed in either tanks or the piping using static mixers.
The final solution may require filtration prior to delivery to reduce the introduction of solids into the delivery wells and the resulting potential for pluggings problems. Simple bag filters or more complex diatomaceous earth filters can be used. A final storage or "head tank" will typically be required because the injection rate may not completely match the instantaneous delivery rate.
The Laramie, Wyoming field demonstration employed two different chemical mixing systems (see Appendix A). For the 1988 pilot study, premixed chemicals were delivered to the site. The solution was filtered through a diatomaceous earth (DE) filter to remove any suspended solids before injection. For the larger 1989 study, concentrated chemicals were delivered to the site and the solutions were mixed on site. Figure 5-17 is a process flow diagram for the solution mix facilities.
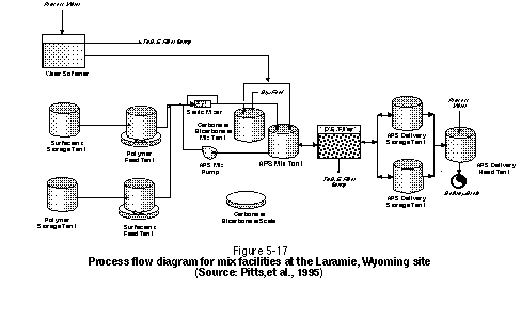
5.5.10 Produced Fluids Treatment and Handling
The range of potential fluids treatment and handling technologies that could be used was discussed in Section 5.3.7. Field demonstrations provide the opportunity to further pilot test treatment methods with larger volumes of produced fluids and with fluids that will be more representative of full-scale operations. However, treatment/handling systems must be in place for the field demonstration to be able to handle the fluids effectively. Thus, one primary treatment/handling and disposal method must be selected based on the laboratory treatability studies. The primary method used may not be the preferred system for full-scale application, but it may have a greater certainty of being effective. The preferred method could be pilot tested to reduce the uncertainty about its effectiveness.
The selection of the primary treatment/handling system must be based on the following criteria:
• The objectives of the demonstration (e.g., recycling of the surfactant)
• The discharge/disposal point
• The permitting and other regulatory requirements of the discharge point
• The certainty in the effectiveness of the system
• The robustness of the system in handling changes in fluid quality and quantity
• The cost of the system
In the pilot tests conducted at Hill AFB OU2, the produced fluids were treated in an existing steam stripper that previously treated high-strength contaminated groundwater from the site. After the steam stripping, the water was discharged to the base WWTP and ultimately to a POTW. The base treatment plant was a physical/chemical system consisting of air stripping, chemical precipitation, and activated carbon treatment. NAPL recovered from the stream stripper was sent off site for incineration.
For the Hill AFB OU1 pilot tests, the majority of the produced fluids were stored in 20,000-gal tanks and gradually pumped to the base (Dyamac, 1996). The base WWTP limited the volume and organic strength loading that it would accept from the field demonstrations. Pilot tests of produced fluids treatment also were conducted on some of the fluids.
During the field demonstrations performed at the Laramie, Wyoming site (see Appendix A), biological treatment was used as the primary treatment method. A pilot study of membrane separation (ultrafiltration) also was conducted. A vendor supplied the pilot membrane system and provided operational support. The membrane process appeared to be relatively effective. The permeate appeared to be acceptable for reuse (after refortification with the surfactant solution). However, the system could not concentrate the oil phase to desirable levels, and the oil phase contained high levels of salts. Operational problems also were encountered. The corrosive nature of the creosote oil resulted in leaks in the seals of the membranes. Pilot studies were not conducted on the handling and disposal of the concentrate produced from the membrane process.
A modified form of activated sludge treatment was used in both phases of pilot tests at the Laramie, Wyoming site. In the first phase, the biological treatment was conducted in two 20,000-gal frac tanks. Foam in the aeration tanks was a reoccurring problem, and anti-foaming agents had to be added to control the foam. For the second phase of demonstrations, a 500,000-gal steel tank was used as an aeration tank to treat a flow of approximately 10 gpm. The long retention time was required because the produced fluid had a very high organic content (typically 10,000 mg/L COD). This organic content was a combination of the surfactant and polymer used and the wood preserving oil recovered. The produced fluids also had a high sodium content from the alkaline agents added to the surfactant. The high sodium content of the fluids complicated the biological treatment by causing dispersion of the biomass so that it would not settle out of the treated liquids. A large amount of alum and ferric c
hloride had to be added to counteract the sodium. The removal of soluble COD was relatively good, with effluent COD values less than 2,000 mg/L (80 percent removal).
5.5.11 Construction and Operation of Field Demonstrations
The construction of field demonstrations can be relatively simple if the size of the demonstration is small, pre-made chemicals are delivered to the site, and the produced fluids are easily treated and handled.
For the Laramie, Wyoming field pilots, the surfactant demonstrations were one of a number of field demonstrations being conducted simultaneously (Sale and Piontek, 1992). Consequently, a general construction contractor was hired to construct the demonstration facilities. Construction included installation of sheet pile walls, installation of injection and recovery drain lines, construction of pumping skids, installation of piping in the 2.3-acre test area, and installation of tanks and supporting mechanical equipment for the chemical makeup system and the produced fluids treatment system.
Operation of a field demonstration requires careful attention, regardless of the scale. However, a large-scale demonstration will require greater manpower to keep the system operating smoothly. Operational tasks include:
• Makeup of surfactant/cosolvent chemical solutions. If pre-made chemicals are used, this could be a relatively simple operation, but mixing raw chemicals is fairly labor intensive.
• Quality control for the chemical solutions. Regardless of how they are supplied, the chemical solutions need to be analyzed on a routine basis to verify that they are consistent with the desired specifications.
• Flow-rate adjustments. Injection flow rates will require adjustment throughout the field demonstration.
• Performance and operations monitoring. This is described in the next section.
• Produced fluids treatment/handling. Operations and monitoring of these systems could be relatively simple or complex, depending on the system involved.
• Ongoing Process Review. The project engineers and hydrogeologists should review the process frequently to verify that the demonstration is being performed as planned and that all required contingencies are being implemented.
5.5.12 Performance and Operations Monitoring
Results and conclusions reached through field demonstrations are based on performance monitoring. General categories of performance monitoring include:
• Initial condition
• Quality of delivered fluids
• Subsurface hydraulics
• Quality of produced fluids
• Performance of mechanical systems
• Endpoint condition
Each of these topics is reviewed below.
Initial Condition
To evaluate the results of the field demonstration, the initial condition should be characterized using the following parameters to establish a baseline.
Mass of Target Compound(s) in the Target Zone. Common performance parameters include the volume of mass recovered and the fraction of total mass recovered. To estimate these parameters, it is necessary to evaluate the initial mass in place. As described in Section 5.2.1, this can be done by a variety of methods.
Aqueous Concentration of Target Compound(s). Often, the goal of in-situ remediation is to improve groundwater quality by reducing the aqueous concentration of target compounds in the groundwater. Where this is the goal, the initial concentration of the target compound should be measured. Because pumping can strongly affect aqueous concentrations, these should be measured under a reasonable background flow condition (e.g., natural flow conditions) that can be duplicated at the end of the test.
Inorganic Water Quality. Many chemical systems currently being considered involve optimization using salinity. Where salts may be used, a baseline condition including major anions and cations should be defined. This will assist in defining an endpoint for aquifer reconditioning, which may be necessary following chemical flushing.
Quality of Delivered Fluids
Bulk chemicals used in field-scale activities may differ significantly from materials used in laboratory-scale work. Biological activity, reactions with piping and tank components, and field mixing of components all may lead to significant deviation. Throughout field activities, monitoring needs to be performed to verify the composition of delivered fluids.
Subsurface Hydraulics
The delivery and cycling of chemical systems creates the potential to adversely affect the hydraulic capacity of the delivery systems and/or the permeability of the target zone. Fluid levels and flow rates should be monitored at representative locations throughout the duration of a field demonstration. These data can be used to monitor any adverse plugging of delivery systems (e.g., well or drain line) or the subsurface target.
Losses in overall delivery capacity often reflect plugging at or near the delivery system. Near-delivery losses can be differentiated from losses in target zone permeability by installing a piezometer in the immediate vicinity of the delivery system. For example, a small-diameter pipe can be placed in the gravel envelope around wells or drain lines during construction. For a given flow, increases in the head loss between the delivery system and the surrounding filter pack typically indicate near-delivery system plugging. Similarly, piezometers in the target zone may be used to characterize the gradient required to move a given flow as a function of time and, thereby, assess plugging within the target zone.
Understanding where and when hydraulic losses occur is an important step in identifying measures to mitigate adverse losses. Factors potentially leading to losses in hydraulic capacity include:
• High levels of suspended solids in the delivered fluids
• Delivered fluids cascading into the delivery system and entraining air
• Formation of insoluble inorganic precipitates
• Formation of stable high-viscosity emulsions
• Dispersion of clays through cation exchange
• Mobilization of clays and other colloids by surfactants
• Growth of biomass
Quality of Produced Fluids
Monitoring produced fluids enables assessment of the following parameters:
Mass Removal. The mass recovered can be estimated by measuring the rate of fluid production and the concentration of target compounds in the produced fluid. This method often leads to a more accurate estimate than using pre- and post-flushing soil cores or tracer tests. At a minimum, produced fluids data can provide a valuable check on mass reduction estimates using in-situ mass measurement methods.
Chemical System Performance. Monitoring flow rates and chemical system concentrations over time can provide useful insights into the in-situ performance of the chemical system. It also allows estimates to be made of the fraction of delivered chemical that was recovered.
Loading to Treatment Systems. Produced fluids data are a critical input to assessing the performance of produced fluids treatment systems. In addition to typical contaminant parameters, this may require monitoring for BOD, COD, hydrocarbon content, and suspended solids.
Produced Fluids Treatment Systems. Treated fluid streams (e.g., air, water, and NAPL) need to be characterized to determine if they meet reuse, discharge, and disposal criteria. Operational monitoring of the treatment system should be conducted as well. The specifics of operational monitoring will depend on the particular technologies employed and discharge standards.
Performance of Mechanical Systems
Field demonstrations provide an important opportunity to test mechanical systems such as pumps, piping, valves, meters, tanks, mixers, blowers, level controllers, flow controllers, and filters. For example, this testing may include:
• Inspection of materials for compatibility with fluids
• Observation of fluids for production of mechanical emulsions
• Validation of the performance of flow meters and level controllers
• Inspection of pumps, pipes, and tanks for adverse wear
Endpoint Condition
At the conclusion of the field demonstration, the endpoint condition must be characterized by repeating, in a comparable fashion, the steps taken to characterize the initial condition. In addition, sediment samples may be analyzed for concentration of chemical system components to confirm the estimates of remaining system components made using produced fluids data.
5.5.13 Reporting
The final results of the field demonstration will need to be compiled and documented in a final report. The format for this report will depend on the requirements of the sponsoring party and the regulatory groups. The EPA has a suggested format for final reports as well as for work plans. The EPA has also recently published guidelines for the presentation of data from remediation technologies (EPA, 1995). Presenting information and data following these guidelines should aid in future comparisons between remediation technologies.
5.5.14 Limitations of Field Demonstrations
Field demonstrations will provide a significantly better indication of the potential performance of a full-scale system compared to a laboratory study or numeric simulations. However, there are limitations to the data obtained from the field demonstration. For example, the location of the field demonstration may be physically and chemically different than other areas of the site where contamination is present. Consequently, the performance of the full-scale system may differ from the field demonstration in some parts of the site.
5.6 Full-Scale Implementation
Relevance
If the field demonstration yields positive results and re-comparison to other alternatives suggests it is the most feasible approach (refer to Figure 5-1) ), the next step is to proceed to site-wide application. The implementation of a full-scale system will follow basically the same steps as discussed in Section 5.5 for field demonstrations. To avoid repeating much of Section 5.5, this section highlights the aspects of full-scale implementation that are different from small-scale field demonstrations. Please note that, to date, surfactant/cosolvent flushing has had no known full-scale application. Therefore, this discussion draws on experience gained from full-scale implementation of other remediation technologies. Readers interested in implementing a surfactant/cosolvent flushing project should read this section.
Key Points
• For sites that are relatively large, it may be prudent to implement a full-scale system through a phased modular approach.
• Performance monitoring for a full-scale system is typically less rigorous than for a field demonstration.
• The design of a full-scale system will differ from a field demonstration because of potential differences in duration, and less flexibility is typically provided in a full-scale systems.
• More permits may be required for a full-scale system compared to a field demonstration.
• Project delivery (i.e. contracting) and staffing for operations are likely to be different for full-scale compared to field demonstrations.
5.6.1 Site-Wide Versus Phased Modular Implementation
Surfactant/cosolvent flushing could be implemented in a single, site-wide step throughout a given target area or in phased modules across the target area. Conditions where each of these approaches has the greatest applicability are discussed below.
Site-Wide Implementation
At small sites, where the size of the full-scale delivery/recovery system is essentially the same as the target area, it is most practical to implement site-wide flushing in a single step. Site-wide implementation is also the preferred approach when full-scale operations need to be completed in the shortest possible time. For almost all other circumstances, phased modular implementation should be considered.
Phased Modular Implementation
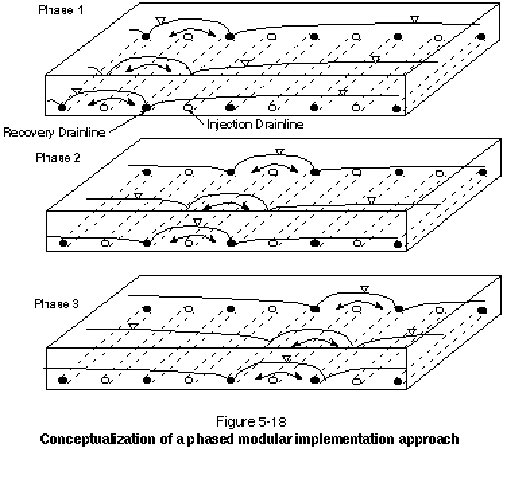
Figure 5-18 illustrates phased implementation of surfactant/cosolvent flushing through a given target zone using parallel sets of delivery and recovery drainlines. Phased approaches also can be implemented using wells. At any given site, the most practical approach to phasing implementation will depend on the chemical system, fluid treatment system, and site characteristics.
A phased modular approach provides the following potential advantages at large sites:
• Design capacities for delivery systems, pumps, piping, and treatment systems can be minimized by addressing a fraction of the target area at any given time.
• Equipment requirements can be minimized by reusing the same equipment for each module.
• Reusing the same chemicals in each progressive module (if appropriate) minimizes chemical costs.
• Committing operations staff to a longer-term project can help to reduce labor costs through workload leveling, reduced mobilization costs, and lower labor rates.
• Lessons learned during operations of the first module(s) can be applied to subsequent modules, leading to ongoing optimization of systems and operations.
• Overall project costs can be distributed over a longer period of time, thus reducing present value costs.
5.6.2 Performance Monitoring
In proceeding from field demonstrations to full-scale implementation, performance monitoring goals shift from developing and testing the technology to documenting process performance. Typically, full-scale monitoring is less intensive than monitoring for demonstrations.
For example, having already documented performance in field demonstrations, and perhaps having verified that performance in the first full-scale module(s), implementation monitoring could be simplified in the following ways:
• Limit analysis of delivered solutions to simple indicator parameters such as viscosity, density, and turbidity.
• Rely solely on concentrations of produced fluids to document mass recovery.
• Define an endpoint for mass recovery and potential aquifer reconditioning based on delivery and recovery of a fixed number of pores and/or asymptotic rates of cumulative recovery.
• Limit fluid-level monitoring to frequencies necessary to define optimal delivery recovery rates.
• Limit analyses of produced fluids and treated waste streams to those parameters needed to make operational decisions and comply with reuse, discharge, and/or disposal criteria.
5.6.3 Full-Scale Design Objective
As with performance monitoring, design objectives change when moving from field demonstrations to full-scale implementation. Field demonstrations are often over-designed in terms of operational flexibility and overall reliability. This approach provides a high potential for success in the face of large uncertainties. Once a field demonstration proves successful, these uncertainties are greatly reduced. Capital costs can then be reduced by, for example, using lower-cost materials, reducing process options, or eliminating unused process components.
Full-scale design must address cost-effective management of the entire produced fluids stream. Produced fluids from field demonstrations can be collected for pilot testing and then disposed of off site. While disposal costs of the entire produced fluids stream are reasonable for small-scale systems, they will almost always be prohibitively high for wastes from large full-scale applications.
5.6.4 Permitting
The permitting required for a full-scale application of surfactant/cosolvent flushing may also be different than for a field demonstration. For example, regulatory agencies may allow small field demonstrations and pilot studies to proceed without formal permits, but they may require permits for full-scale implementation. Permits may include air discharge permits and UIC permits.
5.6.5 Project Delivery
Field demonstrations typically involve a limited amount of work and require a flexible work force. Therefore, contracts for construction of field demonstration facilities are often based on time and materials. In contrast, large full-scale implementation involves significantly larger amounts of both time and materials and requires a minimal number of change orders. These are often lump sum contracts awarded to the lowest qualified bidder (see Chapter 6 for further information on contracting and design).
Because field demonstrations are often short duration, equipment is often rented and systems are designed for short-term operation. Purchasing the equipment necessary for full-scale implementation may provide a significant cost savings over the longer term. Furthermore, where operations may continue for a number of years, systems need to be designed to last through the anticipated project life.
5.6.6 Operations Staff
Field demonstrations are generally performed by a combination of design engineers and scientists (typically consultants) and temporary operations staff. Design engineers and scientists are involved because they are familiar with project objectives, able to troubleshoot problems, and need to be fully familiar with system performance so they can optimize subsequent designs. Full-scale operations are typically staffed by full-time professional operators under the guidance of a project supervisor. The reduced involvement of engineers and scientists once the system is operational reflects both cost concerns and a reduced need for technical expertise.
Forward to Chapter 6
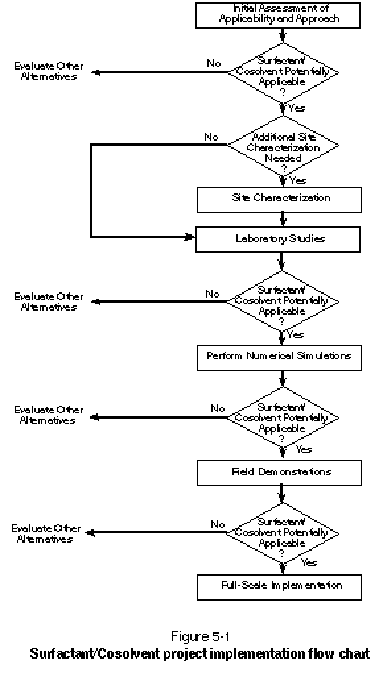
![]() (1)
(1)
![]() (2)
(2)
![]() (3)
(3)