Accelerated Solvent Extractor (ASE) Device

Solid Phase Extraction (SPE)
SPE Process
- Select packing which will absorb compounds of interest
- Select solvent which will elute compounds of interest, but not contaminants
- Sample may require pH adjustment
- Retain compounds of interest on the packing
- Elute the contaminants
- Elute the compounds of interest
Typically, 15 mL of a water sample are extracted using a specific SPE cartridge for the analytes of interest. The cartridges contain silica gel-based, bonded phase packings. Solutions are passed through these tubes by using either vacuum or positive pressure. Samples can be prepared either for HPLC or GC.
For a solid phase extractor, first, condition the tube to activate the tubing. Condition solvents depend on the tube packing. Next, add aliquot of sample and wash. Rinse the packing with a small volume (usually 200 L to 2 mL) of a solution that removes the compounds of interest.
Column types and suppliers
- Extract-Clean™ by Alltech Associates, Inc.
- B&J Solid Phase System by Burdick & Jackson, Division of Baxter
- Accuband® by J & W Scientific
- BAKERBOND SPE by J.T. Baker, Inc.
- Supelclean by Supelco, Inc.
- Bond-Elut® by Varian Sample Preparation Products
- Clean Screen® by Worldwide Monitoring
There are numerous manufacturers of SPE cartridges and supplies. The target compound must be known to select the appropriate cartridge.
Extraction time equals approximately 1 to 2 minutes per step
Used for extraction of pesticides, PCBs, explosives, triazine herbicides, hydrocarbons, phthalate esters, and phenols
Solid Phase Extraction Principles
Solid phase extraction utilizes the same analyte/sorbent interactions that are exploited in the powerful separation technique of high performance liquid chromatography (HPLC). Bond Elut extraction cartridges are packed with a variety of surface-modified bonded silica sorbents which selectively retain specific classes of chemical compounds from a given matrix. As an example, the Bond Elut strong cation exchanger (SCX) can be used to retain the cationic drug, amphetamine, from urine. The more specific the interaction between the sorbent and analyte, the cleaner the final extract.
Bonded silica sorbents are in many ways the ideal materials for chromatographic isolation, primarily due to the number of different functional groups that can be readily bonded to the silica surface. In addition, bonded silicas are rigid supports that do not shrink or swell; possess very large surface areas due to porosity; are stable under a wide range of aqueous and organic solvent conditions; and form a clean, non-leachable substrate upon which the bonded functional groups are attached.
Solid phase extraction consists of four basic steps
The
most common goals of an extraction protocol are clean-up, concentrate, and
solvent exchange (e.g., aqueous to organic) prior to analysis. Solid phase
extraction achieves these goals in four simple steps. They are: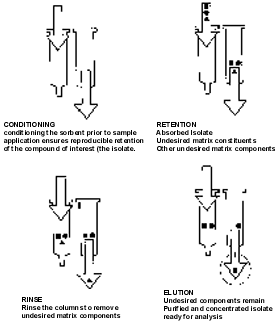
Conditioning: Preparing the cartridge for reproducible interaction with the sample matrix by solvating the sorbent bed. This is done by passing a volume of an appropriate solvent through the cartridge, followed by a volume of a liquid similar in nature to the sample matrix. A common example of cartridge conditioning would be to pass methanol, followed by water, through a C18 cartridge prior to extraction of an aqueous sample matrix.
Retention: Applying the sample to the conditioned cartridge results in the analyte, and perhaps other matrix components, being retained on the sorbent surface due to one or more specific chemical interactions (e.g. Van der Waals or "non-polar" interactions between the hydrocarbon chain of a C18 bonded phase). It should be pointed out that the matrix contaminants may pass through the cartridge unretained, hence cleaning up the sample even at the retention or loading step.
Rinsing: Passing solvents through the cartridge rinses away additional interfering compounds while leaving the analyte undisturbed within the sorbent bed. A common rinse solvent for a non-polar extraction on a C18 sorbent would be water.
Elution: Passing an appropriate solvent through the cartridge which is specifically chosen to disrupt the analyte-sorbent interaction, resulting in selective elution of the analyte. To use a non-polar extraction example again, an organic solvent such as methanol would be a sufficiently strong solvent to disrupt the interaction between most non-polar analytes and a C18 bonded phase.
Thermal Desorption
Desorption can be achieved using solvents such as hexane, trichlorethylene, carbon disulphide, and chloroform. The resulting aliquot is analyzed by GC. For example, Perkin-Elmer has an automatic thermal desorption instrument, the ATD50, which can analyze up to 50 sample tubes in a run. Sampling may be done by pumped or diffusive sampling of volatiles from gas, liquid, or solid samples; direct analysis of solid samples; or direct adsorption of liquid samples placed in a sample tube. These tubes are then placed in a desorption oven where thermal desorption is carried out either by a single or two-stage process. The vapors are passed directly onto the column in the single stage method, whereas in the latter process, the desorbed vapors are collected in an electrically cooled trap and concentrated.
Supercritical Fluid Theory
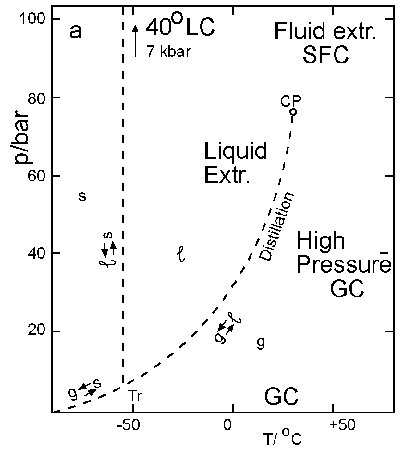
Carbon dioxide is the most frequently used supercritical solvent. The critical point of carbon dioxide is (304.21 Kevin, 73.825 bar, 0.466 grams per centimeter cubed) the point at which the vapor pressure and temperature are such that the solvent is in both the liquid and gas phase. At this point, the vapor density and the liquid density are equal. At any temperature above the critical point only one phase exists, the supercritical phase.
Supercritical fluid extraction (SFE) process
- Process by which a fluid phase having intermediate properties between a gas and a liquid affects the solubilities of solutes
- Supercritical fluid solvents have lower viscosities and higher diffusivities, which allows a more efficient transfer of solutes from sample matrices Gas Chromatography Supercritical Fluid Theory (continued)
- The solubility of fluids can be adjusted by mechanical compression of the extraction fluid
- Allows extraction at lower temperatures, which could be advantageous for temperature sensitive compounds that break down
Solubility of fluids can be adjusted. Lower extraction temperatures could lead to less breakdown of temperature sensitive compounds.
Possible supercritical solvents
- Ethylene, carbon dioxide, nitrous oxide, propane, methanol, water, ammonia, n-pentane
A disadvantage of this method is that it is specific to each group of analytes and it requires tremendous method development.
| Extraction Type | Matrix | Analytes | SW-846 Method Number | Advantages for | Limitations for Field Use |
| Liquid-Liquid | Aqueous | SVOCs | 3510C, 3520C |
•Rigorous method •Proven method •Accepted method |
•High
solvent use •Labor intensive •Long extraction times •Glassware use/cleaning |
| Soxhlet | Solids | SVOCs | 3540C, 3541 | •Rigorous method •Proven method •Accepted method | •High
solvent use • Long extraction times •Glassware use/cleaning |
| Ultrasonic | Solids | SVOCs | 3550B | •Rigorous method •Proven method •Shorter extraction times than soxhlet | •High
solvent use • Potential breakdown of some analytes • Expensive equipment • Less efficient for nonpolar analytes |
| Abbreviated Solvent | Aqueous and Solids | SVOCs | None |
•Rapid |
•Method
development time •Interferences, normally no cleanup steps • Not as rigorous • Can be labor intensive |
| ASE | Solids | PAHs, PCBs, Pesticides | 3545 | •Rapid •Less solvent use •Efficient • Accepted, proven method |
•Expensive extraction apparatus •Logistical constraints (power and space) |
| MAE | Solids | TPH, PAHs, PCBs, Pesticides | None | •Rapid
•Less solvent use •Efficient |
•Expensive
equipment • Logistical constraints (power and space) • Safety concerns • Not accepted yet for organics |
| SFE | Solids | SVOCs | 3560, 3561 | •Rapid
and efficient •No solvent waste • Proven method for some analytes |
•Expensive
equipment • Logistical constraints (not portable) •Sample size is limited • Optimization for different analytes |
| SPE | Aqueous | SVOCs and VOCs | 3535 | •Simple
• Rapid • Uses small volumes of solvent • Inexpensive |
•Dirty
samples may plug disks or cartridges • Choosing the correct sorbent •Analyte breakthrough • Can be labor-intensive |
| SPME | Aqueous, Soil Headspace, Air | SVOCs and VOCs | None | •Simple
and rapid • No solvent use •Can be used for VOCs and SVOCs • Inexpensive |
•Not yet an EPA-accepted method •Can be labor-intensive unless expensive automated equipment is rented or purchased |
| Thermal Desorption | Solids | TPH, PAHs, PCBs | 8275 | •Rapid •Simple •No solvent use |
•Expensive
apparatus |