Dense Nonaqueous Phase Liquids (DNAPLs)
Chemistry and Behavior
- Overview
- Policy and Guidance
- Chemistry and Behavior
- Environmental Occurrence
- Toxicology
- Detection and Site Characterization
- Treatment Technologies
- Conferences and Seminars
- Additional Resources
Halogenated Alkenes
Trichloroethene
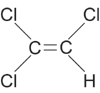 Trichloroethene (TCE, CAS # 79-01-6) is a volatile organic compound that has a high vapor pressure (69 mm Hg at 25°C), is sparingly soluble (1,100 mg/L), and has a specific gravity of 1.464. The log Kow ranges from 2.29 to 3.24 (different references), and the log Koc from 1.81 and 2.10 (Montgomery and Welkom 1991). The Agency for Toxic Substances and Disease Registry has developed a Toxicological Profile for Trichloroethylene that contains a useful summary table
Trichloroethene (TCE, CAS # 79-01-6) is a volatile organic compound that has a high vapor pressure (69 mm Hg at 25°C), is sparingly soluble (1,100 mg/L), and has a specific gravity of 1.464. The log Kow ranges from 2.29 to 3.24 (different references), and the log Koc from 1.81 and 2.10 (Montgomery and Welkom 1991). The Agency for Toxic Substances and Disease Registry has developed a Toxicological Profile for Trichloroethylene that contains a useful summary table![]() of the physical properties of TCE as well as fate and transport information.
of the physical properties of TCE as well as fate and transport information.
When released to the air, TCE has a five to seven-day residence time and degrades to phosgene, dichloroacetyl chloride, and formyl chloride (Howard 1991). TCE has a relatively high Henry's constant (9.85 x 10-3 atm-m3/mole) and will form a vapor plume in the vadose zone above a dissolved phase plume, which can be tracked using soil gas measurement techniques and may present soil vapor intrusion problems for buildings.
Releases to the ground result in either evaporation or percolation into the subsurface. TCE is not expected to bind strongly with soil particles or bioaccumulate. TCE can co-metabolically degrade naturally in an aerobic environment but this is unusual. Under anaerobic conditions, TCE slowly biodegrades via reductive dechlorination. The extent and rate of degradation is dependent upon the strength of the reducing environment, and if the appropriate microbes are not available, the degradation may stall at 1,2-dichloroethene.
TCE that is dissolved in surface water volatilizes with a half life of minutes to hours depending upon the water's energy. Using the above Henry's constant and an estimation method, PubChem reports volatilization half lives for a model river and model lake as 3.5 hours and five days, respectively.
Additional information on TCE is available through the TCE section of Contaminant Focus.
For Further Information
![]() BIOCHLOR: Chlorinated Solvent Plume Database Report
BIOCHLOR: Chlorinated Solvent Plume Database Report
Aziz, C.E., A.P. Smith, C.J. Newell, and J.R. Gonzales
Air Force Center For Environmental Excellence (AFCEE), 78 pp, June 2000
This database of chlorinated solvent plume characteristics was compiled to identify key characteristics of parent and daughter chlorinated solvent plumes and to determine important relationships between plume characteristics and hydrogeologic and environmental variables. The results are intended to provide site managers with general plume length information that is needed to estimate the likelihood of off-site migration and the potential effectiveness of natural attenuation for plume management.
![]() Fate and Transport Modeling of Selected Chlorinated Organic Compounds at Hangar 1000, U.S. Naval Air Station, Jacksonville, Florida
Fate and Transport Modeling of Selected Chlorinated Organic Compounds at Hangar 1000, U.S. Naval Air Station, Jacksonville, Florida
U.S. Geological Survey, Water Resources Investigations Report 03-4089, 1999
Contact: J. Hal Davis, hdavis@usgs.gov
This report discusses the application of a ground water solute transport model for estimating the time of travel for TCE, dichloroethene, and vinyl chloride (VC) to arrive at a discharge point. TCE was estimated to move the slowest and VC the fastest.
Handbook of Environmental Fate and Exposure Data for Organic Chemicals, Volume II Solvents
Howard, P. (ed.)
Lewis Publishers, Chelsea, MI, 1991, 546 pp
This book provides physical and chemical properties and fate and transport information for 82 chemical solvents.
![]() Intermedia Transfer Factors for Contaminants Found at Hazardous Waste Sites: Trichloroethylene (TCE), Final Draft Report
Intermedia Transfer Factors for Contaminants Found at Hazardous Waste Sites: Trichloroethylene (TCE), Final Draft Report
Risk Science program, Dept. of Environmental Toxicology, Univ. of California, Davis;
Office of Scientific Affairs, Dept. of Toxic Substances Control; and California Environmental Protection Agency. 33 pp, 1994.
Groundwater Chemicals Desk Reference
Montgomery, J. and L. Welkom.
Lewis Publishers, Chelsea, MI, 1991, 640 pp
This book provides a summary of physical and chemical properties of a variety of chemicals, including TCE.
Natural Attenuation of Chlorinated Solvent Ground-Water Plumes Discharging into Wetlands
Lorah, Michelle M., David R. Burris, and Linda Jo Dyer.
U.S. Geological Survey Scientific Investigations Report 2004-5220, 203 pp, 2005.
This report describes a study that compares the extent of natural attenuation of chlorinated solvents at the Aberdeen Proving Ground wetland site in Maryland to an inland forested bog in the Colliers Mills Wildlife Management Area near McGuire Air Force Base, NJ, and to demonstrate and compare different methods of sampling and analysis for collecting the site data needed to evaluate natural attenuation in wetlands.
NIOSH Pocket Guide to Hazardous Chemicals
National Institute of Occupational Safety and Health
This guide contains information on the chemical physical properties of TCE and their physical hazards.
![]() Toxicological Profile for Trichloroethylene
Toxicological Profile for Trichloroethylene
Agency for Toxic Substances and Disease Registry
U.S. Department of Health and Human Services, 1997, 335 pp
This profile provides information on human health effects, fate and transport, production, and uses of TCE.
![]() Trichloroethene Sorption to Wetland Soils and Lignitic Sediments from the Northern Gulf Coastal Plain
Trichloroethene Sorption to Wetland Soils and Lignitic Sediments from the Northern Gulf Coastal Plain
Fryar, A., C. Sweat, and J. Sachleben
Eleventh Annual V. M. Goldschmidt Conference, 2001.
This study reports on the kinetics of TCE sorption in a wetlands at the Department of Energy's Paducah, KY, gaseous diffusion plant. It concludes that the less polar the organic substrate is the more sorption occurs.
Trichloroethylene Reduction Pathway Map
Whittaker, Mark, David Monroe, Dong Jun Oh, and Sean Anderson.
The University of Minnesota Biocatalysis/Biodegradation Database.
This document is a diagram of degradation pathways for TCE by different microbial organisms.




