Dense Nonaqueous Phase Liquids (DNAPLs)
Chemistry and Behavior
Halogenated Alkenes
1,1-Dichloroethene
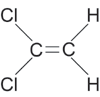 1,1-Dichloroethene (1,1-DCE, CAS # 75-35-4) is a highly volatile and flammable chemical. However, it is heavier than air, and therefore its vapors tend to collect in low areas after a spill. Montgomery and Welkom (1991) estimate the log Koc of 1,1-DCE as 1.81, the log Kow as 2.13 or 1.48 (different sources), and the Henry's constant as 0.021 atm-m3/mole. The solubility of 1,1-DCE is about 400 mg/L at 20° C and its specific gravity is 1.22. The Agency for Toxic Substances and Disease Registry has developed a Toxicological Profile for 1,1-Dichloroethene that contains a useful summary table
1,1-Dichloroethene (1,1-DCE, CAS # 75-35-4) is a highly volatile and flammable chemical. However, it is heavier than air, and therefore its vapors tend to collect in low areas after a spill. Montgomery and Welkom (1991) estimate the log Koc of 1,1-DCE as 1.81, the log Kow as 2.13 or 1.48 (different sources), and the Henry's constant as 0.021 atm-m3/mole. The solubility of 1,1-DCE is about 400 mg/L at 20° C and its specific gravity is 1.22. The Agency for Toxic Substances and Disease Registry has developed a Toxicological Profile for 1,1-Dichloroethene that contains a useful summary table![]() of the physical properties of 1,1-DCE.
of the physical properties of 1,1-DCE.
The low calculated Koc value for 1,1-DCE in soil indicates that 1,1-DCE is likely to move quickly through soil and sediment.
The relatively high Henry's constant value indicates a propensity for 1,1-DCE to volatilize out of water. This property has two implications for environmental remediation. When 1,1-DCE is in a dissolved-phase ground water plume that discharges into a moving body of water, it is very likely to volatilize relatively quickly. Information on PubChem suggests that the half life of 1,1-DCE in a one-meter deep river is about three hours, and in a lake, the half life is about four days. The second implication is that 1,1-DCE may be very susceptible to air sparging as a cleanup technology when subsurface conditions permit.
Because of the low Kow of 1,1-DCE, bioaccumulation is not expected to occur to any significant extent. 1,1-DCE can be degraded by microbial action under aerobic and anaerobic conditions. While it is more easily degraded under anaerobic conditions, the necessary microbial species may not be present in some aquifers. For more information see the Treatment Technologies section.
When released to the atmosphere, hydroxyl radicals are expected to degrade 1,1-DCE with a half life of 1.5 days. Under photochemical smog situations with nitrogen dioxide present the half life falls to less than two hours. Degradation products of 1,1-DCE in the presence of nitrogen oxides include chloroacetyl chloride, phosgene, formaldehyde, formic acid, hydrochloric acid, carbon monoxide, and nitric acid (PubChem).
1,1-DCE is reactive with aluminum, sunlight, air, copper, and heat (Note: polymerization may occur if exposed to oxidizers, chlorosulfonic acid, nitric acid, or oleum. Inhibitors, such as the monomethyl ether of hydroquinone, are added to prevent polymerization). The NIOSH Pocket Guide to Hazardous Chemicals contains more information on the chemical physical properties of 1,1-DCE.
For Further Information
Concise International Chemical Assessment Document 51: 1,1-Dichloroethene (Vinylidene Chloride)
International Programme on Chemical Safety II.Series
World Health Organization
This document is a comprehensive review of the physical/chemistry properties, fate and transport, human and ecological health effects, and occurrence of 1,1-DCE.
Groundwater Chemicals Desk Reference
Montgomery, J. and L. Welkom.
Lewis Publishers, Chelsea, MI, 1991, 640 pp
This book provides a summary of physical and chemical properties of a variety of chemicals, including 1,1-DCP.
NIOSH Pocket Guide to Hazardous Chemicals
National Institute of Occupational Safety and Health
This guide contains information on the chemical and physical properties of 1,1-DCE and its physical hazards.
![]() Toxicological Profile for 1,1-Dichloroethene
Toxicological Profile for 1,1-Dichloroethene
Agency for Toxic Substances and Disease Registry (ATSDR)
U.S. Department Of Health And Human Services, 1994, 204 pp
This profile provides information on human health effects, fate and transport, production, and uses of 1,1-DCE.





