Per- and Polyfluoroalkyl Substances (PFAS)
Chemistry and Behavior
- Overview
- Policy and Guidance
- Chemistry and Behavior
- Occurrence
- Toxicology
- Site Characterization and Analytical Methods
- Remediation Technologies
- Conferences and Seminars
- Additional Resources
Perfluorooctanoic Acid (PFOA)
Perfluorooctanoic acid (PFOA) is a perfluoroalkyl carboxylic acid. Figure 1 shows the basic PFOA Structure—a 7-carbon fully fluorinated aliphatic backbone and a carboxylic acid functional group.
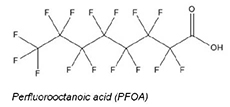
Figure 1. PFOA Structure
Source: SGP 2015
PFOA is a strong acid that is generally present in solution as perfluorooctanoate anion. It is water soluble and mobile in water. PFOA is stable in environmental media because it is resistant to environmental degradation processes, such as biodegradation, photolysis, and hydrolysis. In water, no natural degradation has been demonstrated, and dissipation is by advection, dispersion, and sorption to particulate matter. PFOA has low volatility in ionized form but can adsorb to particles and be deposited on surface soil and into water bodies (USEPA 2016).
Field evidence of PFOA biomagnification, the preferable metric for assessing bioaccumulation potential (Gobas et al. 2009), has been documented in many organisms from many locations worldwide (UNEP 2015) as cited in USEPA 2016. The weight of evidence for trophic magnification was deemed sufficient to consider PFOA to be bioaccumulative by the Stockholm Convention on Persistent Organic Pollutants Review Committee (UNEP 2015), as cited in USEPA 2016.
Table 1. Physical Chemical Properties of PFOA |
||
|---|---|---|
| Property | Perfluorooctanoic Acid | Source |
| Chemical Abstracts Service Registry No. (CASRNa) | 335-67-1 | |
| Chemical Abstracts Index Name | 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8- Pentadecafluorooctanoic acid |
|
| Synonyms | PFOA; Pentadecafluoro-1-octanoic acid; Pentadecafluoro-n-octanoic acid; Octanoic acid, pentadecafluoro-; Perfluorocaprylic acid; Pentadecafluorooctanoic acid; Perfluoroheptanecarboxylic acid; |
|
| Chemical Formula | C8HF15O2 | |
| Molecular Weight (g/mol) | 414.09 | HSDB (2012); Lide (2007); SRC (2016) |
| Color/Physical State | White powder (ammonia salt) | HSDB (2012); Lewis (2004) |
| Boiling Point | 192.4°C; Stable when bound | HSDB (2012); Lide (2007); SRC (2016) |
| Melting Point | 54.3°C | HSDB (2012); Lide (2007); SRC (2016) |
| Vapor Pressure | 0.525 mm Hg at 25°C (measured) 0.962 mm Hg at 59.25°C (measured) |
Hekster et al. (2003); HSDB (2012); SRC (2016) ATSDR (2015); Kaiser et al. (2005) |
| Henry's Law Constant | Not measurable | ATSDR (2015) |
| pKa | 2.80 | SRC (2016) |
| Koc | 2.06 | Higgins and Luthy (2006) |
| Kow | Not measurable | ATSDR (2015); EFSA (2008) |
| Solubility in Water | 9.50 x 103 mg/L at 25°C (estimated) | ATSDR (2015); Hekster et al. (2003); HSDB (2012); Kauck and Diesslin (1951); SRC (2016) |
| Half-life in Water (25°C) | Stable | UNEP (2015) |
| Half-life in Air | Stable when bound | UNEP (2015) |
| Notes: Kow = octanol-water partition co-efficient; Koc = organic carbon-water partitioning coefficient; g/mol = grams per mole aThe CASRN given is for linear PFOA, but toxicity studies are based on a mixture of linear and branched; thus, the RfD applies to the total linear and branched. Source: USEPA 2016 |
||
References
Gobas, F.A.P.C., W. de Wolf, L.P. Burkhard, E. Verbruggen, and K. Plotzke. 2009. Revisiting bioaccumulation criteria for POPs and PBT assessments. Integrated Environmental Assessment and Management 5(4):624-637.
SGP (Scientific Guidance Panel). 2015. ![]() Potential Designated Chemicals: Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs). California Environmental Contaminant Biomonitoring Program, Meeting of March 13, 2015, 22 pp.
Potential Designated Chemicals: Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs). California Environmental Contaminant Biomonitoring Program, Meeting of March 13, 2015, 22 pp.
UNEP (United Nations Environmental Program). 2015. Proposal to List Pentadecafluorooctanoic Acid (CAS No: 335-67-1, PFOA, Perfluorooctanoic Acid), Its Salts and PFOA-Related Compounds in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants. UNEP/POPS/POPRC.11/5.
USEPA (U.S. Environmental Protection Agency). 2016. ![]() Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). Office of Water, EPA 822-R-16-005, 103 pp.
Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). Office of Water, EPA 822-R-16-005, 103 pp.
![]() Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA)
Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA)
U.S. EPA, Office of Water.
EPA 822-R-16-005, 103 pp, 2016
The document offers an overview in chapter 2 of PFOA physical and chemical properties and environmental fate.
Perfluorooctanoic Acid
Hazardous Substances Data Bank, 2016 Update
HSDB contains information on a wide variety of topics related to PFOA, including physical-chemical properties and environmental fate and exposure.
![]() PFOA Isomers, Salts and Precursors: Literature Study and Evaluation of Physico-Chemical Properties
PFOA Isomers, Salts and Precursors: Literature Study and Evaluation of Physico-Chemical Properties
Nielsen, C.J., University of Oslo, Klif project no. 3012013, 68 pp, 2012
In addition to physical-chemical data tables for a variety of PFOA-related compounds, this report provides an extensive list of references.
Proposal to List Pentadecafluorooctanoic Acid (CAS No: 335-67-1, PFOA, Perfluorooctanoic Acid), Its Salts and PFOA-Related Compounds in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants
United Nations Environmental Program, UNEP/POPS/POPRC.11/5, 18 pp, 2015
This overview document contains information on PFOA-related use, chemical structure, physiochemical properties, persistence, bioaccumulation, potential for long-range environmental transport, and adverse effects.
![]() Screening Assessment Report: Perfluorooctanoic Acid, Its Salts, and Its Precursors
Screening Assessment Report: Perfluorooctanoic Acid, Its Salts, and Its Precursors
Environment Canada and Health Canada, 97 pp, 2012
This overview document discusses PFOA-related physical-chemical properties, uses, environmental fate (including the presence of precursors in atmospheric samples), persistence, bioaccumulation, and ecological and human risk.
Sources, Fate and Transport of Perfluorocarboxylates
Prevedouros, K., I.T. Cousins, R.C. Buck, and S.H. Korzeniowski.
Environmental Science & Technology 40(1):32-44(2006) [Abstract]
This review describes the sources, fate, and transport of perfluorocarboxylates (PFCAs) in the environment, with a specific focus on perfluorooctanoate (PFO), the disassociated ion of PFOA. Additional information: Supporting Information.![]()





