- Evaluating Performance of the Monticello PRB in Treating Uranium and Metals
- Residual Waste Mixtures Tailored for Restoration of Vegetative Cover in Metals-Contaminated Soil
- Molasses-Based Microbial Precipitation Used Successfully for Chromium Reduction
- Long-Term Monitoring of Co-Solvent Flooding at Sage's Dry Cleaners
- ITRC Offers Web-Based Training on PRB Installations
Evaluating Performance of the Monticello PRB in Treating Uranium and Metals
Since construction of a permeable reactive barrier (PRB) at Monticello, UT, in July 1999, the U.S. Department of Energy (DOE) has closely evaluated performance of the PRB’s zero-valent iron (ZVI) reactive medium. Evaluation has involved a range of investigative methods, including tracer studies, colloidal boroscope measurements, hydraulic conductivity tests, a large-scale pump drawdown test, and an extensive coring program. The 100- by 8-ft PRB was installed to treat ground water containing uranium, selenium, vanadium, and other metals at concentrations that exceeded maximum contaminant levels (MCLs) by as much as a factor of 100 (see June 2000 Ground Water Currents). During the first year of operation, results of the evaluation indicated that contaminant concentrations were reduced to non-detectable levels in ground water passing through the system. Four years of monitoring results indicate that concentrations of contaminants in ground water exiting the PRB remain below compliance levels and in many cases are lower than instrument detection limits (Figure 1).
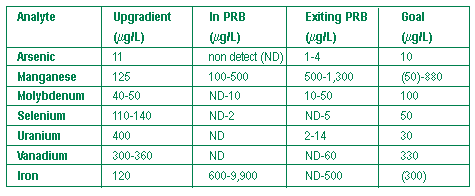
The PRB is constructed with an upgradient zone containing 2 feet of ZVI (13% by volume) mixed with pea gravel (the gravel/ZVI zone) and a downgradient zone containing 4 feet of 100% ZVI (the ZVI zone). Monitoring data taken at points of ground water exit from the gravel/ZVI zone indicate that the average concentration of uranium increased from 0.2 to 185 µg/L after 2.7 years of operation. The increased concentrations indicate that this portion of the PRB is becoming progressively less effective in containing uranium. Concentrations of uranium a short distance into the ZVI zone, however, remain less than the MCL (30 µg/L). Manganese and iron con-centrations are increasing within the PRB but are buffered within several feet of exiting it.
Tracer tests involving upgradient injection of bromide and iodide were conducted to determine flow paths through the PRB. These tests indicated that some ground water flows along preferential paths that are not perpendicular to the PRB. Tracer tests also indicated that residence times in the PRB range from 22 to 90 hours. Additional evaluation of ground-water flow velocities was conducted using a colloidal boroscope (downhole video camera). Boroscope measurements indicated variable flow directions and velocities ranging from 5 to 42 ft/day.
Gas injection slug tests in the ZVI zone showed it has a hydraulic conductivity of approximately 0.07cm/sec—about seven times that of the alluvial aquifer. To further establish the rate of ground-water flow, ground water was pumped through a subsurface horizontal well constructed within the PRB’s air sparging zone to draw down the water table. The water table within the PRB drew down uniformly, which indicated a high rate of hydraulic conductivity. Based on the steady-state pumping rates, ground-water flow within the PRB was estimated to range from 6 to 9 gal/min.
In February 2002, after 2.7 years of operation, 70 cores were collected from the reactive media in the gravel/ZVI and ZVI zones. Analytical results for 279 samples collected randomly indicate that more than 9 tons of calcium carbonate and 24 kg of combined uranium- and vanadium-bearing minerals had deposited in the PRB. Nearly all the precipitated uranium and vanadium was collected in the gravel/ZVI zone. Analysis of the spatial distribution of the precipitates indicated that all portions of the PRB along its length had treated uranium and vanadium. Calcium was identified throughout both the gravel/ZVI and ZVI zones, indicating that precipitation rates of calcium carbonate are slower than uptake rates for uranium and vanadium. Solid-phase chemistry data were used in combination with dissolved-phase ground-water chemistry to determine an average ground-water flow rate of about 5 gal/min.
To help evaluate the nature of the PRB corrosion process, core samples were examined with an electron microprobe. ZVI grains in the gravel/ZVI zone were found to be corroded, but much of the original ZVI remained. The encountered corrosion products included various mixtures of iron oxides and carbonates that formed ZVI grain replacements and coatings, as well as separate aggregates. Specialized test methods (based on gas evolution upon reaction with dilute hydrogen chloride) were used to determine the amount of reactive ZVI in a sample. Preliminary results indicate that much of the ZVI still exists in the 100% ZVI zone, but that much of the ZVI in the gravel/ZVI zone has been lost to oxidative corrosion.
A second round of coring, tracer testing (by borehole dilution), and gas-injection slug testing is scheduled for Summer 2003. These tests are designed to determine if hydraulic conductivity of the PRB is decreasing and to evaluate the rate of uranium uptake by the PRB. Selected core samples will be examined using fission-track methods to determine the distribution of uranium in the mineral framework. In addition, preliminary laboratory tests will be conducted to determine if chemical removal of corrosion products such as calcium carbonate can improve PRB efficiency. Operation of the PRB is expected to continue until 2015, at which time the plume is expected to move beyond the PRB.
Contributed by Richard Muza, EPA/Region 8 (303-312-6595 or muza.richard@epa.gov), Paul Mushovic EPA/Region 8 (303-312-6662 or mushovic.paul@epa.gov), and Dr. Stan Morrison, S.M. Stoller Corp. (970-248-6373 or stan.morrison@gjo.doe.gov)
Residual Waste Mixtures Tailored for Restoration of Vegetative Cover in Metals-Contaminated Soil
Researchers from the U.S. Department of Agriculture (USDA), University of Washington, and U.S. EPA Environmental Response Team (ERT) have collected data over three growing seasons on metals-contaminated soil that was treated in 1997-1998 at the Bunker Hill, ID, Superfund site. Amendments consisting of various mixtures of municipal biosolids, woody debris, wood ash, pulp and paper sludge and compost were added to surface soil and waste material [see May 1998 Tech Trends]. In addition to reducing the bioavailability of metals in surface and subsurface soil (to 1.5 ft), the treatment was anticipated to help correct soil pH, reduce erosion, and restore plants. Results indicate that surface application of high-nitrogen biosolids combined with high calcium carbonate residual such as wood ash can effectively establish a vigorous plant cover directly atop metal mine tailings.
The treatment site encompasses 6-8 acres of mountainous terrain that were barren as a result of past mining activities (Figure 2). Prior to treatment, the site’s surface consisted of heterogeneous lead- and zinc-contaminated waste soil with little to no organic matter and low nutrient status. Chemical nutrients and organic materials were hand mixed with the surface soil/materials in extremely high volumes: 44-66 tons/acre of nitrogen-containing biosolids and 220 tons/acre of wood ash, with or without 44 tons/acre of sludge or log yard debris (20% by volume).

All amendment mixtures were able to restore the vegetative cover for the three years in which data were collected. One year after amendment, a plant biomass of 0.01 tons/acre was measured in a control plot and conventionally treated plot (amended by lime and microbial stimulants). In the biosolid plot, however, the biomass had increased to 3.4 tons/acre. After another year, biomass in the test plot had further increased by 25-50%. Cross-treatment comparisons indicated that amendments containing high nitrogen-content biosolids yielded higher biomass, while those including log yard debris yielded no change in biomass.
Analysis of carbon/nitrogen ratios produced similar findings. All of the amendment combinations resulted in carbon/nitrogen ratios that approached the status of a well-functioning soil system. The carbon/nitrogen ratio generally decreased from approximately 40:1 prior to treatment to a relatively stable 20:1.
In both the control and conventionally treated plots, subsoil pH ranged from 5.6 to 7.0. In plots amended with biosolid combinations, subsoil acidity decreased significantly (to pH levels reaching 8.2) but at different rates. Treatment involving high nitrogen biosolids combined with ash was found more effective than low-nitrogen/ash treatment. In addition, small but significant increases in pH were achieved by using more reactive ash and by including log yard debris. Increases in pH generally corresponded with reduced concentrations of [Ca(NO3)2-]-extractable (and bioavailable) zinc in subsurface soil.
Zinc, lead, and cadmium concentrations in soil and waste materials at the site ranged as high as 14,700, 27,000, and 28 mg/kg, respectively, prior to amendment. However, bioavailability of these metals decreased as much as 50% within 3 years of amendment application. [For additional information on extensive bioavailability studies conducted by the USDA, go to http://www.nps.ars.usda.gov.] Although metal concentrations in plant tissue have remained within normal since the time of soil amendment, concentrations of nutrients such as calcium, potassium, and manganese decreased somewhat. Further research is needed to assess the cause of this nutrient reduction and to evaluate additional combinations of residual waste amendment.
Monitoring of the soil and vegetation systems is anticipated to continue over the next five years. High costs for implementing this technology at the Bunker Hill site are attributed to the expense of purchasing biosolid material, which is in great demand in timber-producing areas such as central Idaho. Preliminary success at this site has lead to similar applications at mining sites in Jasper County, MO, and Leadville, CO.
Contributed by Sally Brown, University of Washington (206-616-1299 or slb@u.washington.edu) and Harry Compton, EPA/ERT (732-321-6751 or compton.harry@epa.gov)
Molasses-Based Microbial Precipitation Used Successfully for Chromium Reduction
Carbohydrate solution (dilute blackstrap molasses and water) was injected directly into a metals-contaminated shallow aquifer on a daily basis for 46 months at the Avco Lycoming Superfund site in Williamsport, PA. Rapid degradation of the molasses carbohydrates by indigenous heterotrophic microorganisms helped establish anaerobic in-situ reactive zones that promoted a metal reduction process (Figure 3). Monitoring indicates that use of this metals precipitation system resulted in nearly complete reduction of hexavalent chromium to less soluble (and less toxic) states such as trivalent chromium.
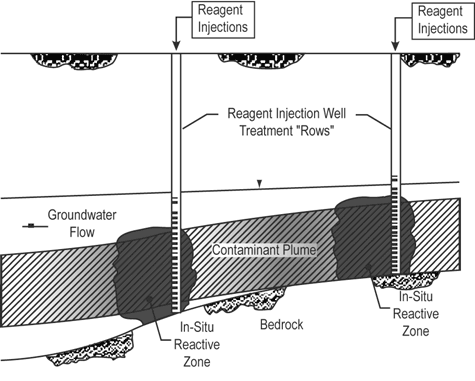
The project cleanup goals for hexavalent chromium and dissolved cadmium are 0.032 mg/L and 0.003 mg/L, respectively. Hexavalent chromium and dissolved cadmium concentrations prior to treatment reached a maximum of 2.29 mg/L and 0.26 mg/L, respectively. Preliminary data collected after 18 months of treatment indicated that significant microbial reduction had occurred at the 12,000-ft2 treatment area [see December 1998 Ground Water Currents]. The treatment system was terminated in November 2000, when six rounds of quarterly data analysis indicated that 12 of the treatment area’s 14 monitoring wells had exceeded performance criteria and that no contaminant rebound had occurred. By that time, hexavalent chromium concentrations had decreased nearly 99%, and dissolved cadmium concentrations had decreased approximately 62%.
Monitoring data collected over the past two and a half years indicate that metal concentrations in all eight of the treatment wells have not varied significantly since the time of system shut-off. The most recent data indicate that concentrations in six of the treatment wells meet the cleanup goals for hexavalent chromium and dissolved chromium. In the remaining wells, hexavalent chromium concentrations decreased 75-99% from pre-treatment levels, and dissolved cadmium concentrations decreased 10-62%. No evidence of metal migration has been found in any of the system’s eight downgradient monitoring wells, where cleanup goals continue to be met.
A five-year review performed by EPA in July 2002 concluded that the metals precipitation system, combined with a pump and treat system focused on hydrocarbon "hot spots," will significantly reduce the time needed to achieve site cleanup. Semi-annual collection of ground-water data will continue through 2003.
The Environmental Security Technology Certification Program/Air Force Center for Environmental Excellence has initiated demonstrations of this technology for treating chlorinated hydrocarbon-contaminated ground water at the former Hanscom Air Force Base, Vandenburg Air Force Base, former Naval Station at Treasure Island and Badger Army Ammunition Plant. Field studies also are underway to evaluate the technology’s performance in addressing sites with contaminants of recent and widespread concern, such as perchlorate and 1,4-dioxane, and other dissolved metals and uranium.
Contributed by Jill Lowe, EPA/Region 3 (215-814-3123 or lowe.jill@epa.gov)
Long-Term Monitoring of Co-Solvent Flooding at Sage's Dry Cleaners
Co-solvent flooding was conducted in 1998 at an abandoned site known as Sage's Dry Cleaners in Jacksonville, FL, to remediate perchloroethene (PCE)-contaminated ground water (see June 1999 Ground Water Currents). Early results of the project indicated that the maximum and minimum observed rates of PCE dechlorination were approximately 43.6 and 4.2 µg/L/day, respectively (see June 2000 Ground Water Currents). Since that time, the University of Florida has continued its efforts to monitor the site’s ground water on a long-term basis. The monitoring program is anticipated to help assess the long-term impact of co-solvent flooding, including the extent to which residual ethanol is enhancing microbial degradation of PCE.
The monitoring program interprets mass flux leaving the source zone as an early response to remediation. Ground-water samples extracted from a network of wells and multilevel samplers are analyzed periodically to determine the changing concentrations of PCE and its degradation components, and to estimate the complete electron mass balance. Samples are collected from two multilevel networks: (1) a set of seven multilevel samplers, each with five levels for a total of 35 sampling points, to assess the source zone; and (2) a transect of three multilevel downgradient samplers to assess the early plume response.
Prior to the co-solvent flood, the average PCE concentration in the multilevel source zone sampler was about 49 mg/L (Figure 4). Approximately one year was required for the co-solvent flood response to reduce these concentrations to about 26 mg/L. This length of time was attributed to the need for natural gradient ground-water flow to displace fluid remaining after the flood operations. PCE concentrations were found to vary in accordance with differing patterns between the flow of co-solvent flood and the natural gradient flow. In addition, residual ethanol may have influenced PCE concentrations in the source zone. In the multilevel transect downgradient of the source zone, the response time exceeded two years.
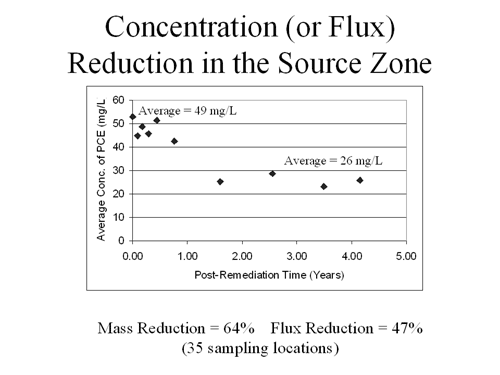
The observed 47% reduction in PCE concentrations in the source zone (Figure 4) suggests that the mass flux leaving the source zone has decreased approximately the same amount (assuming that ground-water velocity and direction have not changed significantly). Based on a mass removal of approximately 64%, these results indicate that a flux reduction of 47% was achieved. Monitoring also has revealed significant generation of degradation byproducts that were not present prior to co-solvent flooding, which suggests that residual ethanol has promoted further degradation of PCE. (Ethanol concentrations averaged approximately 1% within the contaminant source zone upon completion of the flooding.) Investigators estimate that the PCE plume response resulting from the co-solvent flood will require years or decades for realization.
Contributed by Michael Annable, University of Florida (352-392-3294 or annable@ufl.edu)
ITRC Offers Web-Based Training on PRB Installations
On July 24, 2003, the Interstate Technology Regulatory Council (ITRC) will host a two-hour training course on various techniques to design, install, maintain, and monitor iron-based PRBs. As a follow-up to earlier ITRC training, this course will focus on case studies involving long-term performance of iron-based PRB systems, and will address the mechanisms employed in PRBs based on non-iron materials. Participants may register for the session at http://www.cluin.org/live.
Elements of the course are based on three documents developed by the ITRC's Permeable Reactive Barriers Team and the Remediation Technologies Development Forum: Regulatory Guidance for Permeable Barrier Walls Designed to Remediate Chlorinated Solvents (2nd ed., PBW-1, 1999), Regulatory Guidance for Permeable Reactive Barriers Designed to Remediate Inorganic and Radionuclide Contamination (PRB-3, 1999), and Design Guidance for Application of Permeable Barriers to Remediate Dissolved Chlorinated Solvents (PBW-2, 2000) (available at http://www.itrcweb.org).






