For more information on Nanomaterials, please contact:
Technology Integration and Information Branch
PH: 202-566-0875 | Email: adam.michael@epa.gov
Nanotechnology: Applications for Environmental Remediation
Application
Since the early 1990s, site project managers have taken advantage of the properties of metallic substances such as elemental iron to degrade chlorinated solvent plumes in groundwater. One example of an in situ treatment technology for chlorinated solvent plumes is the installation of a trench filled with macroscale zero-valent iron (ZVI) to form a permeable reactive barrier (PRB) (ITRC 2005). Based on the success of using macroscale metallic substances for environmental remediation, nanoscale materials are being researched and applied as in situ contamination reduction technologies. Following are brief descriptions of nanoscale materials for environmental remediation.
Nanoscale Materials
Most of the bench-scale research and field application of nanoscale materials for remediation at full scale has focused on nanoscale zero-valent iron (nZVI) and related products. However, other nanoscale materials, such as titanium dioxide (TiO2), are in the research and development stages for use in environmental remediation (see Nanotechnology Products with Potential Remediation Applications).
Nanoscale zero-valent iron (nZVI): Particles of nZVI may range from 10 to 100 nanometers in diameter or slightly larger. Macroscale ZVI has been shown to be effective for treating groundwater contaminants within PRBs. Particles of nZVI provide the same environmental remediation benefits as macroscale ZVI but have larger surface areas per volume of material; this larger surface area provides more reactive sites allowing for more rapid degradation of contaminants when compared to macroscale ZVI (U.S. EPA 2008B). A disadvantage of nZVI is the agglomeration or clumping of particles to each other or to the soil surface. Agglomeration may be caused by groundwater conditions, surface properties of the particles, the age of the materials, or shipping conditions (U.S. EPA 2007A). Modifications to nanoscale iron particles have been made to enhance their mobility, reactivity, or stability. Examples include adding coatings such as polyelectrolyte or triblock polymers (Saleh et al. 2007; Hydutsky et al. 2007; He et al. 2007) to improve mobility, or encasing in emulsified vegetable oil droplets (Quinn et al. 2005) to improve stability and to improve reactivity by improving contact with the contaminant (see EZVI section below). Some nanoscale materials are made with catalysts (see Bimetallic nanoscale particles section below) that enhance the intrinsic reactivity of the surface sites (Tratnyek and Johnson 2006). It should be noted that modifications to enhance mobility may result in reduced reactivity (Phenrat et al. 2009).
Bimetallic nanoscale particles (BNP): BNPs have been used to remediate contaminants in soil and groundwater. BNPs consist of particles of elemental iron or other metals in conjunction with a metal catalyst, such as platinum, gold, nickel, and palladium. The combination of metals increases the kinetics of the oxidation-reduction (redox) reaction, thereby catalyzing the reaction. Palladium and iron BNPs are commercially available and currently the most common. In bench-scale tests, BNPs of iron combined with palladium achieved contaminant degradation two orders of magnitude greater than microscale iron particles alone: these particles were 99.9 percent iron and less than 0.1 percent palladium (Zhang and Elliot 2006). Palladium can catalyze the direct reduction of trichloroethene (TCE) to ethane without producing other intermediate by-products such as vinyl chloride (Nutt et al. 2005). BNPs are generally incorporated into a slurry for injection and can be injected by gravity or by pressure feed (Gill 2006).
Figure 2. Structure of an EZVI particle (modified from O'Hara et al. 2006)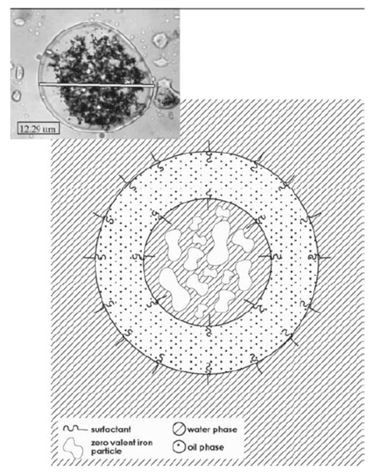
![]()
Emulsified zero-valent iron (EZVI): EZVI is commercially available and has been used to remediate chlorinated solvents. EZVI consists of nano- or microscale ZVI surrounded by an emulsion membrane that facilitates treatment of chlorinated hydrocarbons. The exterior emulsion membrane is made from food-grade surfactant and biodegradable oil and the inside of the droplets contain water and the ZVI particles. Figure 2 illustrates the structure of an EZVI particle. The exterior emulsion membranes are hydrophobic, similar to the properties of dense nonaqueous phase liquid (DNAPL) contaminants such as TCE. EZVI particles (or "droplets") therefore mix directly with DNAPL. When the emulsion droplets come into contact with with a TCE DNAPL, the TCE partitions into the oil membrane and then diffuses into the interior of the emulsion droplet, where it comes into contact with the ZVI and is degraded. A concentration gradient is established by migration of the TCE molecules into the interior aqueous phase of the emulsion droplet and by migration of the by-products out of the droplet and into the surrounding water phase, further driving the degradation reactions (O'Hara et al. 2006). The vegetable oil can also provide "food" (electron donors) to microorganisms and enhance biological activity, which in turn contributes to the destruction of the contaminant (Quinn et al. 2005). In addition, EZVI can be especially effective when DNAPL is present because DNAPL tends to be miscible in vegetable oil. When DNAPL contacts EZVI, the DNAPL can mix with the EZVI, after which the contaminants are in close proximity with the ZVI and can be effectively degraded.
Contaminants Known to be Treated Using Nanoscale Materials
Nanoscale Iron
Research indicates that nanoscale materials such as nZVI, BNPs, and EZVI may chemically reduce the following contaminants effectively: tetrachloroethene (PCE), TCE, cis-1,2-dichloroethylene (c-DCE), vinyl chloride (VC), and 1-1-1-tetrachloroethane (TCA), polychlorinated biphenyls (PCBs), halogenated aromatics, nitroaromatics, metals such as arsenic and chromium (U.S. EPA, 2008A), and nitrate, perchlorate, sulfate, and cyanide (Tratnyek et al., as cited in Tarr 2003).
Two of the most important reductive dechlorination reaction mechanisms are hydrogenolysis and beta elimination. Beta elimination, which occurs most frequently when the contaminant comes into direct contact with elemental iron (Fe0), follows the degradation pathway below:
TCE + Fe0 → Chlorinated Acetylene → Hydrocarbon Products + Cl- + Fe2+/Fe3+ (U.S. EPA, 2008A).
The hydrogenolysis pathway for reductive dechlorination of PCE, which occurs under the reducing conditions fostered by nZVI in groundwater, follows the following degradation pathway:
PCE → TCE → DCE → VC → ethene (Tratnyek et al., as cited in Tarr 2003; U.S. EPA 1999).
The reactions of Fe0 with contaminants may be especially complicated, involving multiple competing pathways.
Other Nanoscale Materials
Nanoscale TiO2 has been shown to mineralize a variety of herbicides, insecticides, and pesticides via photocatalysis and can convert other contaminants to less toxic compounds (Konstantinou and Albanis 2003).
Reactive Chemistry of Nanoscale Materials
Nanoscale Iron/Iron
Zero-valent, or elemental, iron is a reducing reagent that can react with both dissolved oxygen (DO) and water (Zhang 2003). The chemical processes of nZVI are driven by the oxidation of Fe0. However, the exact transformation mechanism may involve competing pathways and depends on the nature of the contaminant and targeted media (Dickinson 2010). In the presence of an oxidizing agent, Fe0 becomes oxidized to ferrous ions (Fe2+), and the two released electrons become available to reduce other compounds. In aerobic conditions, Fe0 reacts with dissolved oxygen to form ferrous ions and water. Fe0 can also reduce water to form ferrous ions, hydrogen, and hydroxide ions. These reactions are shown below:
2Fe0 + 4H+ + O2 →2Fe2+ + 2H2O
(Matheson and Tratnyek 1994)
2Fe0 + 2H2O →2Fe2+ + H2 + 2OH-
(Matheson and Tratnyek 1994)
The reactions of Fe0 with contaminants (such as TCE) may be more complicated and may involve multiple competing pathways, including beta elimination, where Fe0 releases electrons and TCE is transformed to ethane and releases chloride ions. The equations above and these reactions apply to iron particles in general; however, the advantages of using nanoscale iron over macro- or microscale iron is that reactions may proceed at a faster rate.
The reductive capability of Fe0 when it comes into contact with chromium contamination in soil and groundwater can be seen in the following equation, where iron is oxidized to its ferrous form and chromium is reduced from chromium (VI) to the less toxic chromium (III) (Cao and Zhang 2006):
3Fe0 + 4H2O + 2CrO42- → 3Fe2+ + 2Cr3+ + 8OH-
After groundwater treatment using nanoscale iron particles, post-injection observations of the subsurface have indicated an increase in pH (caused by the formation of hydroxide ions) and a decrease in oxidation-reduction potential (ORP) (a result of the reducing conditions that are created). A lower ORP favors anaerobic bacterial growth, which may promote increased degradation for certain contaminants (for example, chlorinated solvents). Other chemicals formed when particles such as nZVI are used may include hydrogen gas and Fe2+ ions, which also would promote microbial growth. After an nZVI injection, the ORP tends to decrease sharply before it becomes stable (Zhang 2003).
Other Materials
When aqueous TiO2 suspensions are irradiated with light energy greater than 3.2 electronvolts (eV), TiO2 is activated and can combine with water or dissolved oxygen (or both) to form highly reactive species, including the hydroxyl radical and the superoxide radical anion, which can oxidize and degrade a range of contaminants (U.S. EPA 2008A).
In Situ Application of Nanoscale Materials
The application of nanoscale materials for in situ application is site specific. The method of injection, and spacing and distribution of injection points, will depend on the type of geology found in the treatment zone, the type and distribution of contaminants, and the form of the nanoscale materials that will be injected. Injection of nanoscale iron is typically done via direct injection through gravity feed or under pressure. Direct injection can be done via several options including, but not limited to, direct push technology or via various types of wells (e.g., temporary or permanent injection wells). Recirculation is another option that involves injecting nanoscale materials, while extracting groundwater and reinjecting it in the treatment zone, possibly adding more nanoscale materials in the process. This method of in situ application keeps the water in the aquifer in contact with the nZVI and also prevents larger agglomerated iron particles from settling out, promoting continuous contact with the contaminant. Figure 3 shows an example of the recirculation in situ application of nanoscale materials. Another common in situ application method for nZVI is the direct push method. This method involves driving direct-push rods, similar in size to small drilling augers, progressively deeper into the ground. This method allows materials to be injected without the need to install permanent wells (Butler 2000).
Figure 3. Conceptual design of recirculation (Henn 2005)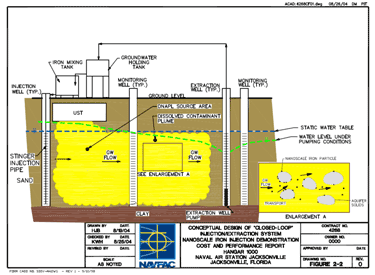
![]()
Additional methods and processes to apply nanoscale material for in situ treatment include pressure pulse technology, liquid atomization injection, pneumatic fracturing, and hydraulic fracturing. Pressure pulse technology uses large-amplitude pulses of pressure to insert the nZVI slurry into porous media at the water table; the pressure then excites the media and increases fluid level and flow (OCETA 2003). Liquid atomization injection is a technology that is proprietary to ARS Technologies, a company that specializes in pneumatic fracturing and injection field services. It introduces an nZVI-fluid mixture into the subsurface using a carrier gas. The nZVI liquid gas combination aerosolizes, allowing for more effective distribution; this method can be used in geologic formations with lower permeability (NAVFAC 2008). Fracturing injection (pneumatic or hydraulic) is a high-pressure injection technique using compressed gas (pneumatic) or a water-based, highly viscous slurry containing sand (hydraulic) that fractures rock or other low-permeability formations and allows liquids and vapors to be transported quickly through the channels created. Pneumatic fracturing uses compressed gas to create a fracture network of preferential flow paths in rock around the injection point to allow liquids and vapors to be transported quickly through the fractured rock; pneumatic fracturing improves access to contaminants and allows liquids to flow freely (Pneumatic Fracturing Inc. 2008; Zhang 2003).
Figure 4 illustrates the basic principles of two methods of remediating contaminated groundwater using nZVI. The image at the top shows treatment of DNAPL contamination by injection of nanoscale materials. In the second image, a reactive treatment zone is formed by a series of injections of nZVI. These injections create overlapping zones of particles that become lodged within the native aquifer material (Tratnyek and Johnson 2006).
Figure 4. Schematic of two methods of groundwater remediation using nanoscale iron (CGR 2009)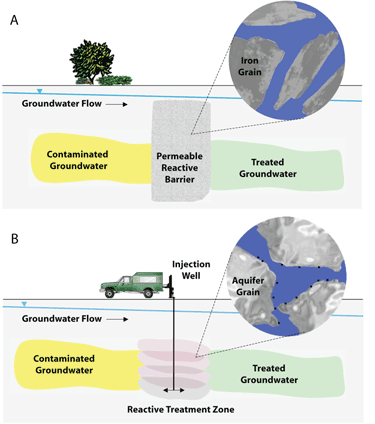
![]()
Research is under way into methods of injection that will allow nanoscale materials to better maintain their reactivity and increase their access to recalcitrant contaminants by achieving wider distribution in the subsurface. For example, creating nZVI on site could reduce the amount of oxidation the iron undergoes between production and use, thereby reducing potential losses in reactivity. Researchers in green chemistry have successfully created nZVI in soil columns using a wide range of plant phenols which, according to the researchers, allows greater access to the contaminant and creates less hazardous waste in the manufacturing process (Varma 2008).
Factors Affecting Performance
A number of factors can affect nanoscale performance for site remediation, including: (1) site specific conditions, (2) agglomeration, and (3) passivation. Each of these is discussed below.
Site-specific conditions such as the site location and layout, geologic conditions, concentration of contaminants, and types of contaminants may limit the effectiveness of nanoscale materials. Before nanoscale materials are injected, geologic, hydrogeologic, and subsurface conditions should be evaluated to assess whether injected particles would offer the performance expected. Factors that affect the subsurface mobility of nanoscale materials include the surface features of nanoscale materials and site-specific factors including: composition of the soil matrix, ionic strength of the groundwater, hydraulic properties of the aquifer, depth to the water table, and geochemical properties (including pH, DO, ORP, and the concentration of nitrate, nitrite, and sulfate), among others. Performance will be site specific and will depend on the presence of competing oxidants such as DO and NO3- (nitrate ion), contaminant concentration, and the pH of the soil and groundwater (Liu and Lowry 2006; Liu et al. 2007).
Agglomeration - Studies have shown that nanoscale materials may not achieve widespread distribution in the subsurface because of agglomeration before they completely disperse within the soil or groundwater matrix, limiting the radius of influence. nZVI particles are attracted to one another, which can cause them to agglomerate into larger micron-sized particles (greater than 100 nanometers) (Tratnyek and Johnson 2006; Phenrat et al. 2007). Because of agglomeration, the effective size might range up to hundreds of nanometers. Some vendors refer to their materials as nanoscale even though they are larger than 100 nanometers and therefore do not meet the NNI definition of nanotechnology.
Agglomeration also reduces the exposed reactive surface area of the particles. The pH of the subsurface may also limit the effectiveness of nanoscale materials because the sorption strength, agglomeration, and mobility of the particles are all affected by the pH of the groundwater (U.S. EPA 2007B). The ionic strength and types of cations in the groundwater, as well as the chemical and physical characteristics of the aquifer materials, also affect the agglomeration and movement of iron nanoscale materials (Saleh et al. 2008).
Passivation, or deactivation, may limit the effectiveness of iron nanoscale materials. The high surface energy of nanoscale materials makes them highly reactive and susceptible to oxidation in the open air, resulting in the formation of iron oxide and a decrease in the chemical reactivity of the iron nanoscale materials (Wang et al. 2010). As a rule, injection mechanisms should limit the volume of water injected along with the iron to limit exposure to oxygen and other oxidants that could passivate the iron before and during injection. If larger volumes are used, deoxygenated water can minimize the iron passivation, but other oxidants may still be present to react with the iron (Gavaskar et al. 2005).
A challenge in evaluating the performance of nanoscale material injection is monitoring the distribution of injected particles in the subsurface. It is therefore important to identify the appropriate parameters to measure performance. Typically, geochemical measures such as ORP are monitored as a surrogate. Dissolved iron can also be monitored. Reaction kinetics are difficult to monitor; however, post-injection chemical concentrations are measured using standard approaches. Additionally, the kinetics and reactivity of nanoscale materials in a DNAPL source zone may vary from the kinetics and reactivity in a dissolved plume (U.S. EPA 2008A; Liu et al. 2007).
Fate, Transport, and Toxicity
Although nanoscale materials that contain iron are the most widely used nanoscale materials in site remediation, knowledge is limited on the fate and transport of iron nanoscale materials in the environment. Furthermore, research is ongoing regarding the potential toxicological effects of nanoscale materials. There are insufficient data on the potential for bioaccumulation of nanoscale materials in environmentally relevant species (Kreyling et al. 2006), and there have been few studies on the effects of any nanoscale materials on environmental microbial communities (Klaine et al. 2008). As described in the "Factors Affecting Performance" section, agglomeration often affects transport of nanoscale materials in the subsurface. The particles may become associated with the aquifer matrix as oxidized iron particles after they react with contaminants. Under standard environmental conditions (aerated water and pH 5 to 9), Fe2+ will readily and spontaneously oxidize to Fe3+ and precipitate out of the groundwater as insoluble iron oxides and oxyhydroxides. Researchers have developed methods, some of which are in use commercially, to improve the mobility of iron nanoscale materials within aquifers and to optimize contact between the nanoscale material and contaminant. Ongoing studies are evaluating surface coatings and other modifications that would reduce agglomeration of nanoscale materials and maximize subsurface mobility (Phenrat 2008). Preliminary research indicates that polymers, surfactants, and organic proteins (such as soy) stabilize nanoscale material suspensions in aquifers, inhibiting their agglomeration and allowing greater dispersal without compromising the ability of the iron to remediate contaminants (He et al. 2007, Zhang 2009). Based on laboratory research, soils high in clay content have been shown to allow greater dispersal of nZVI as well; anionic clay particles appear to function as a natural stabilizer, allowing for more effective transport (Schrick et al. 2004; Hydutsky et al. 2007). Increasing mobility without sacrificing reactivity needs to be tempered with methods to control the migration of the nanoscale particles due to lack of toxicological information (Lowry et al. 2007).
Studies are being conducted on the potential toxicity of various types of manufactured nanoscale material. The increased surface area and larger number of reactive sites of nanoscale materials may equate to greater biological activity per unit mass than with micro- or macroscale particles of the same composition. Substances considered nontoxic at the macroscale may have negative impacts on human health when nanoscale particles are inhaled, absorbed through the skin, or ingested (Kreyling et al. 2006). Because of the small size of nanoscale materials, the particles have the potential to migrate to, or accumulate in, places that larger particles cannot, such as the alveoli in the lungs (Grassian et al. 2007), thereby potentially increasing toxicity. Some nanoscale materials have demonstrated an ability to increase the bioavailability of certain hydrophobic contaminants, for example, by increasing the mobility of contaminants bound to soil and sediment surfaces (Tungittiplakorn et al. 2005). Potential health effects of nanoscale materials have been reviewed in Nanoparticles: Health Effects—Pros and Cons, written by Maureen Gwinn and Val Vallyathan. Issues of toxicity and safety have limited the use of nanoscale materials for remediation by some private-sector companies. For example, DuPont has ruled out the use of nZVI for remediation at its sites until issues concerning fate and transport have been more thoroughly researched. The company has cited questions of post-remediation persistence and potential human exposure to the particles as areas of particular concern (DuPont 2007). As another example of a cautionary approach, the Continental Western Group of insurance companies announced![]() that it will no longer cover injury or damage arising from nanotubes or nanotechnology, as used in products or processes.
that it will no longer cover injury or damage arising from nanotubes or nanotechnology, as used in products or processes.
U.S. Environmental Protection Agency's (EPA) Office of Research and Development (ORD) published a Nanomaterial Research Strategy (NRS)![]() in June 2009 to act as a guide for nanotechnology research within ORD. The NRS centers around progressing EPA's understanding of nanomaterials for decision support and focuses on four research themes:
in June 2009 to act as a guide for nanotechnology research within ORD. The NRS centers around progressing EPA's understanding of nanomaterials for decision support and focuses on four research themes:
- Identifying sources, fate, transport, and exposure
- Understanding human health and ecological research to inform risk assessment and test methods
- Developing risk assessment approaches
- Preventing and mitigating risks (U.S. EPA 2009).
In addition, EPA ORD has prepared a document, Nanomaterial Case Studies: Nanoscale Titanium Dioxide in Water Treatment and in Topical Sunscreen. The document discusses two case studies on the use of nanoscale TiO2: 1) to remove arsenic from drinking water, and 2) as an active ingredient in topical sunscreen. The document was designed to define the information required to perform a comprehensive environmental assessment of the potential risks associated with nanoscale TiO2.
Field Demonstrations and Case Studies
As of November 2011, data that exhibit varying degrees of comprehensiveness were obtained for 36 sites using or testing nanoscale materials for remediation or in the planning stages. Details on these selected sites are available at http://clu-in.org/products/nanozvi and will be updated periodically as new information is received. Performance data are limited because many of the remediation projects using nanoscale materials are just beginning or are ongoing. However, as the technology is applied at an increasing number of sites with varying geologies, more data will become available on performance, providing site managers and other stakeholders with additional information to evaluate whether the technology might be applicable for their sites.
Cost
In 2001, when field demonstrations of nZVI technology first took place, the cost of nZVI was considered high (nearly $500/kilogram [kg]) due to a limited number of commercial suppliers. In addition, the effectiveness and quality of commercially-available nanoscale material products were highly variable when compared to laboratory-prepared nanoscale material due to insufficient characterization and quality assurance/quality control procedures (Li 2006). By 2006, nZVI costs had reduced to about $50 to $100 per kg. Decreasing nZVI costs and improved quality control procedures that provide better homogeneity are making this technology more viable in today's remediation market. While costs may remain high in some applications, the total costs of deploying the technology at a site compared to the life cycle cost of an ex situ technology can be quite competitive.
In addition to the costs associated with the nanoscale material, other factors contribute to the cost of site remediation such as site type, contaminant type, contaminant concentration, extent of the contaminant plume, and any challenges that may occur during remediation. Additional factors that may increase the total cost of nanoscale material application may include operational requirements connected with any contamination found underneath a building, or the need to treat or dispose extracted fluids (Wilson 2004). Currently, project-specific financial information is limited because many of the remediation projects using iron nanoscale materials are just beginning or are ongoing. Proprietary concerns may also preclude cost data from being made publicly available. However, as the use of the technology increases at sites with varying geologies, more cost data will become available, providing site managers and other stakeholders with additional information to evaluate the applicability and cost-effectiveness of the technology for their sites.
Nanotechnology Vendors
The properties of particles such as reactivity, mobility, and shelf life can vary depending on the manufacturing process or the vendor that provides the particle (Miehr et al. 2004). Several vendors supply nanoscale materials for site remediation. See http://clu-in.org/products/nanozvi for information about vendors associated with site-specific applications. More information about vendors and other types of nanoscale materials can be found on the Internet at sites such as http://www.nanovip.com or http://www.nanotech-now.com/business.htm. The inclusion of these websites should not be considered to be an endorsement by EPA.
Nanotechnology Products with Potential Remediation Applications
Researchers are developing a variety of other nanoscale materials for potential use to adsorb or destroy contaminants as part of either in situ or ex situ processes. The stage of development ranges from bench to pilot scale. These particles include TiO2, SAMMSTM, nanotubes, ferritin, dendrimers, metalloporphyrinogens, and swellable organically modified silica (SOMS).
Nanoscale TiO2 is being pilot tested for ex situ treatment of contaminated groundwater as part of a pump and treat system. Additional information on nanoscale TiO2 is available within Contaminants Known to be Treated Using Nanoscale Materials and Reactive Chemistry of Nanoscale Materials.
Some materials can be made with surface functional groups to serve as adsorbents to scavenge specific contaminants from waste streams. SAMMSTM materials consist of a nanoporous ceramic substrate coated with a monolayer of functional groups tailored to preferentially bind to the target contaminant. The functional molecules covalently bond to the silica surface, leaving the other end group available to bind to a variety of contaminants. Though SAMMSTM materials are larger than the nanoscale range, they are considered a nanotechnology because of their nanoscale pores. According to researchers, SAMMSTM maintains good chemical and thermal stability and can be readily reused or restored (Fryxell et al. 2007). SAMMSTM has a large surface area to allow for quick sorption kinetics. Contaminants successfully sorbed to SAMMSTM include radionuclides, mercury, chromate, arsenate, pertechnetate, and selenite (Mattigod 2003; Tratnyek and Johnson 2006). According to the SAMMSTM Adsorbents website, SAMMSTM materials have produced positive results in pilot-scale tests for remediation of mercury in well water with a high concentration of dissolved solids, aqueous mercury in low concentrations, highly radioactive mercuric waste, and gaseous elemental mercury. Since SAMMSTM materials adsorb the contaminants, these materials are used primarily as an ex situ treatment technology (Tratnyek and Johnson 2006).
Nanotubes are engineered molecules most frequently made from carbon. They are electrically insulating, highly electronegative, and easily polymerized. Nanotubes have also been made from TiO2 and have demonstrated the potential for use as a photocatalytic degrader of chlorinated compounds (Chen et al. 2005). Bench-scale research has shown TiO2 nanotubes are particularly effective at high temperatures and are capable of reducing contaminant chemical concentrations by more than 50 percent in 3 hours (Xu et al. 2005). TiO2 is considered an ex situ strategy because effective illumination usually requires that treatment occur in a reactor that is designed for this purpose (Tratnyek and Johnson 2006).
Bench-scale tests using ferritin, an iron storage protein, have indicated that it can reduce the toxicity of contaminants such as chromium and technetium in surface water and groundwater to facilitate remediation (Temple University 2004). Like TiO2, ferritin is photocatalytic; in one bench-scale project, the addition of visible light caused ferritin to reduce toxic, water-soluble hexavalent chromium to the less toxic trivalent chromium, which is not water soluble and precipitates out of solution (U.S. EPA 2008A). Ferritin is being researched for in situ use (Temple University 2004).
Dendrimers are hyper-branched, well-organized polymer molecules with three components: core, branches, and end groups. Dendrimer surfaces terminate in several functional groups that can be modified to enhance specific chemical activity. Fe0/FeS nanocomposites, synthesized using dendrimers as templates, could be used to construct permeable reactive barriers for remediation of contaminated groundwater. Bench-scale research has indicated that delivery options for dendrimers are flexible (Diallo et al. 2006). Dendrimers can be used in situ or ex situ (Xu 2006).
Metalloporphyrinogens are complexes of metals with naturally occurring, organic porphyrin molecules. Examples of biological metalloporphyrinogens are hemoglobin and vitamin B12. Batch-reactor experiments have shown that metalloporphyrins are capable of reducing chlorinated hydrocarbons such as TCE, PCE, and carbon tetrachloride under anoxic conditions to remediate contaminated soil and groundwater. Laboratory research using metalloporphyrinogens is being performed for the potential in situ remediation of groundwater (Dror et al. 2005).
SOMS (commercial name Osorb) is organically modified silica that swells and captures small molecule organic compounds such as gasoline, natural gas, acetone, ethanol, pharmaceuticals, and solvents. When organic compounds are introduced to SOMS, the powder swells, capturing the organic compounds. SOMS may be able to capture up to eight-times its volume in organic compounds that are present either as neat liquids, vapors, or dissolved in water. The SOMS material is hydrophobic and does not absorb water (Edmiston 2009).
- Iron-Osorb incorporates nZVI into Osorb, forming a material that can dechlorinate captured organics. The glass matrix concentrates the chlorinated solvents and protects the embedded metal particles from deactivation by dissolved ions. After the Iron-Osorb has captured the organic compounds, it can be put through a thermal or rinsing process to release the compounds allowing for the capture of the organic compound and the reuse of the Iron-Osorb. Iron-Osorb can go through this regeneration process over 100 times (ABS Materials 2010B). The manufacturer reports that Iron-Osorb material is designed to last over 10 years in the subsurface. For long-term remediation, it can be injected as a soft curtain barrier to protect groundwater areas from incoming plumes. In situ pilot scale testing was performed at several sites in Ohio in 2009 for groundwater remediation of TCE
 .
. - Palladium-Osorb is a similar metal-glass hybrid material used in ex situ treatment systems for remediation of chlorinated volatile organic compounds (VOCs) (Edmiston 2010). Palladium nanoparticles are incorporated into the Osorb to remove halogenated VOCs from groundwater pumped through the system. A hydrogen gas source supplies a proton that completes the reductive dehalogenation of contaminants inside the Palladium-Osorb, and reduces contaminants to benign products of ethane gas and salts (in the reduction of TCE) (ABS Materials 2010A). Pilot scale testing at an Ohio site has shown the continuous reduction of TCE from 5800 parts per million to no detect by the ex situ system. The manufacturer reports that Palladium-Osorb is a catalytic material that does not break down or need replacement. An above ground unit has been designed to treat 10 gallons per minute of produced water (ABS Materials 2010A) and the unit has been modified to be able to treat the oil sheens and mousses that result from oil spills. The unit is being field-tested for possible use in the Gulf of Mexico (Boccieri 2010).
Researchers are also using nanotechnology to develop membranes for water treatment, desalination, and water reclamation. These membranes incorporate a wide variety of nanoscale materials, including those composed of alumina, zero-valent iron, and gold (Theron et al. 2008). Carbon nanotubes can be aligned to form membranes with nanoscale pores to filter organic contaminants from groundwater (Mauter et al. 2008; Meridian Institute 2006).
Citations
ABS Materials. 2010A. Osorb The VOC-Eater™ 10 Web page. Available at: http://www.absmaterials.com/ex-situ-remediation. Accessed August 19, 2010.
ABS Materials. 2010B. Osorb Web page. Available at: http://www.absmaterials.com/osorb. Accessed July 31, 2010.
Boccieri, John. 2010. Boccieri Announces $200,000 To Abs Materials Company, LLC. Available at: http://http://bignews.biz/?id=899733&keys=Congressman-Roy-Blunt-OilSpill. Accessed August 19, 2010.
Butler, J.J., A.A. Lanier, J.M. Healey, and S.M. Sellwood. 2000. Direct-Push Hydraulic Profiling in an Unconsolidated Alluvial Aquifer. Kansas Geological Survey, Open-file Report 2000-62. Available at: http://www.kgs.ku.edu/Hydro/Publications/OFR00_62/index.html. Accessed September 25, 2008.
Cao, J., and W-X., Zhang. 2006. Stabilization of Chromium Ore Processing Residue (COPR) with Nanoscale Iron Particles. Journal of Hazardous Materials. 132(2-3):213-219.
Center for Groundwater Research (CGR). 2009. Zero-Valent Iron (ZVI) Web page. Available at: http://cgr.ebs.ogi.edu/iron/#results. Accessed September 29, 2009.
Chen, Y., J.C. Crittenden, S. Hackney, L. Sutter, and D.W. Hand. 2005. Preparation of a Novel TiO2-Based P-N Junction Nanotube Photocatalyst. Environmental Science & Technology. 39(5):1201-1208.
Diallo, M., P. Hudrlik, and A. Hudrlik. 2006. Fe(0)/FeS Dendrimer Nanocomposites for Reductive Dehalogenation of Chlorinated Haliphatic Compounds: Synthesis, Characterization and Bench Scale Laboratory Evaluation of Materials Performance. Power Point Presentation. Available at: http://www.howard.edu/CEACS/news/HBCU_MI/Howard%20-Diallo.ppt. Accessed September 25, 2008.
Dickinson, M. and T.B. Scott. 2010. The Application of Zero-Valent Iron Nanoparticles for the Remediation of a Uranium-Contaminated Waste Effluent. Journal of Hazardous Materials, 178: 171-179.
Dror,I., D. Baram, and B. Berkowit. 2005. Use of Nanosized Catalysts for Transformation of Chloroorganic Pollutants. Environmental Science & Technology. 39(5):1283-1290.
DuPont. 2007. Nanomaterial Risk Assessment Worksheet, Zero Valent Nano Sized Iron Nanoparticles (nZVI) for Environmental Remediation. Available at: http://www2.dupont.com/Media_Center/en_US/assets/downloads/pdf/NRAW_nZVI.pdf![]() .
.
Edmiston, P.L. 2009. Swellable Glass-Nano ZVI Composite Materials for the Remediation of TCE and PCE in Groundwater. June 12, 2009.
Edmiston, P.L.. 2010. Pilot Scale Testing of Swellable Organosilica-Nanoparticle Composite Materials for the In Situ and Ex Situ Remediation of Groundwater Contaminated with Chlorinated Organics. May 13, 2010. Available at: www.frtr.gov/meetings1.htm.
Fryxell G, S. Mattigod, L. Yuehe, W. Hong, S. Fiskum, K. Parker, Z. Feng, W. Yantasee, T. Zemanian, R. Addleman, L. Jun, K. Kemner, S. Kelly, and F. Xiangdong. 2007. Design and Synthesis of Self Assembled Monolayers on Mesoporous Supports (SAMMS™): The Importance of Ligand Posture in Functional Nanomaterials. Journal of Materials Chemistry. 17:2863-2874.
Gavaskar, A., L. Tatar, and W. Condit. 2005. Cost and Performance Report Nanoscale Zerovalent Iron Technologies for Source Remediation. Naval Facilities Engineering Command (NAVFAC). September 2005.
Gill, H.S. 2006. Bimetallic Nanoscale Particles. PARS Environmental Inc. Power Point Presentation. Available at: http://www.itrcweb.org/Documents/sedimentsgillslides.pdf![]() . Accessed September 25, 2008.
. Accessed September 25, 2008.
Grassian, V.H., P. T. O'Shaughnessy, A. Adamcakova-Dodd, and J.M. Pettibone. 2007. Inhalation Exposure Study of Titanium Dioxide Nanoparticles with a Primary Particle Size of 2 to 5 nm. Environmental Health Perspectives. 115(3):397-402.
He, F., D. Zhao, J. Liu, and C.B. Roberts. 2007. Stabilization of Fe-Pd Nanoparticles with Sodium Carboxymethyl Cellulose for Enhanced Transport and Dechlorination of Trichloroethylene in Soil and Groundwater. Industrial & Engineering Chemistry Research. 46:29-34.
Henn. K. 2005. Nanoscale Iron Injection Demonstration at Hangar 1000. NAVFAC. December 2005.
Hydutsky, B.W., E.J. Mack, B.B. Beckerman, J.M. Skluzacek, and T.E. Mallouk. 2007. Optimization of Nano- and Microiron Transport Through Sand Columns Using Polyelectrolyte Mixtures. Environmental Science & Technology. 41:6418-6424.
Interstate Technology & Regulatory Council (ITRC). 2005. Permeable Reactive Barriers: Lessons Learned/New Directions. PRB-4. 2005. Available at: http://www.itrcweb.org/documents/prb-4.pdf![]() . Accessed October 2, 2008.
. Accessed October 2, 2008.
Klaine, S.J., P.J.J. Alvarez, G.E. Batley, T.E. Fernandes, R.D. Handy, D.Y. Lyon, S. Mahendra, M.J. McLaughlin, and J.R. Lead. 2008. Nanoparticles in the Environment: Behavior, Fate, Bioavailability, and Effects. Environmental Toxicology and Chemistry. 27(9):1825-1851.
Konstantinou, I.K., and T. A. Albanis. 2003. Photocatalytic Transformations of Pesticides in Aqueous Titanium Dioxide Suspensions Using Artificial and Solar Light: Intermediates and Degradation Pathways. Applied Catalysis B: Environmental. 42:319-335.
Kreyling, W.G., M. Semmler-Behnke, and W. Möller. 2006. Health Implications of Nanoparticles. Journal of Nanoparticle Research. 8:543-562.
Li, Xiao-qin, D.W. Elliot, and W. Zhang. 2006. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Critical Reviews in Solid State and Materials Sciences 31: 111 - 122. Available at: http://www.site.gitechllc.net/uploads/Cri_Rev_2006.pdf![]() . Accessed March 13, 2011.
. Accessed March 13, 2011.
Liu, Y., and G.V. Lowry. 2006. Effect of Particle Age (Fe0 Content) and Solution pH on NZVI Reactivity: H2 Evolution and TCE Dechlorination. Environmental Science & Technology. 40(19):6085-6090.
Liu, Y., T. Phenrat, and G.V. Lowry. 2007. Effect of TCE Concentration and Dissolved Groundwater Solutes on NZVI-Promoted TCE Dechlorination and H2 Evolution. Environmental Science & Technology. 41(22):7881-7887.
Lowry, G.V., S.A. Majetich, K. Matyjaszewski, and R.D. Tilton. 2007. Final Report: Developing Functional Fe(0)-Based Nanoparticles for In Situ Degradation of DNAPL Chlorinated Organic Solvents. National Center for Environmental Research Web site. Available at: http://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract/6346/report/F. Accessed May 15, 2009.
Matheson, L.J., and P.G. Tratnyek. 1994. Reductive Dehalogenation of Chlorinated Methanes by Iron Metal. Environmental Science & Technology. 28(12):2045-2053. Accessed September 25, 2008.
Mattigod, S.V., G.E. Fryxell, R.J. Serne, and K.E. Parker. 2003. Evaluation of Novel Getters for Adsorption of Radioiodine from Groundwater and Waste Glass Leachates. Radiochim Acta. 91:539-545.
Mauter, M.S., and M. Elimelech. 2008. Environmental Applications of Carbon-Based Nanomaterials. Environmental Science & Technology. 42(16):5843-5859.
Meridian Institute. 2006. Overview and Comparison of Conventional Water Treatment Technologies and Nano-Based Treatment Technologies. Background Paper for the International Workshop on Nanotechnology, Water and Development, 10-12 October 2006, Chennai, India.
Miehr, R., P.G. Tratnyek, J.Z. Bandstra, M.M. Scherer, M.J. Alowitz, and E.J. Bylaska. 2004. Diversity of Contaminant Reduction Reactions by Zerovalent Iron: Role of the Reductate. Environmental Science & Technology. 38(1):139-147.
NAVFAC. 2008. Environmental Restoration Technology Transfer (ERT2) Web page, Nanoscale Zero Valent Iron Tool. Available at: http://t2.serdp-estcp.org/printfriendly.aspx?tool=zvit. Accessed September 25, 2008.
Nutt, M.O., J.B. Hughes, and M.S. Wong. 2005. Designing Pd-on-Au Bimetallic Nanoparticles for Trichloroethylene Hydrodechlorination. Environmental Science & Technology. 39(5):1346-1353.
O'Hara, S., T. Krug, J. Quinn, C. Clausen, and C. Geige. 2006. Field and Laboratory Evaluation of the Treatment of DNAPL Source Zones Using Emulsified Zero-Valent Iron. Remediation Journal. Spring 2006.
Ontario Centre for Environmental Technology Advancement (OCETA). 2003. Pressure Pulse Technology (PPT) for Recovery of Nonaqueous Phase Liquids. OCETA Environmental Technology Profiles. 2003.
Quinn, J., C. Geiger, C. Clausen, K. Brooks, and C. Coon, S. O'Hara, T. Krug, D. Major, W.-S. Yoon, A. Gavaskar, and T. Holdsworth. 2005. Field Demonstration of DNAPL Dehalogenation Using Emulsified Zerovalent Iron. Environmental Science & Technology. 39(5):1309-1318.
Phenrat, T., N. Saleh, K. Sirk, R. Tilton, and G.V. Lowry. 2007. Aggregation and Sedimentation of Aqueous Nanoiron Dispersions. Environmental Science & Technology. 41(1):284-290.
Phenrat, T., N. Saleh, K. Sirk, H. Kim, R. Tilton, and G. Lowry. 2008. Stabilization of Aqueous Nanoscale Zerovalent Iron Dispersions by Anionic Polyelectrolytes: Adsorbed Anionic Polyelectrolyte Layer Properties and Their Effect on Aggregation and Sedimentation. Journal of Nanoparticle Research. 10:795-814.
Phenrat, T., Y. Liu, R. Tilton, and G.V. Lowry. 2009. Adsorbed Polyelectrolyte Coatings Decrease Fe0 Nanoparticle Reactivity with TCE in Water: Conceptual Model and Mechanisms. Environmental Science & Technology. 43(5): 1507-1514.
Pneumatic Fracturing, Inc. Pneumatic Fracturing. 2008. Available at: http://www.pneumaticfracturinginc.com/pneumatic.html. Accessed September 25, 2008.
Saleh, N., K. Sirk, Y. Liu, T. Phenrat, B. Dufour, K. Matyjaszewski, R. Tilton, and G. Lowry. 2007. Surface Modifications Enhance Nanoiron Transport and NAPL Targeting in Saturated Porous Media. Environmental Engineering Science. 24(1):45-57.
Saleh, N., H-J. Kim, T. Phenrat, K. Matyjaszewski, R.D. Tilton, and G. Lowry. 2008. Ionic Strength and Composition Affect the Mobility of Surface-Modified Fe0 Nanoparticles in Water-Saturated Sand Columns. Environmental Science & Technology. 42:3349-3355.
Schrick, B., B.W. Hydutsky, J.L. Blough, and T.E. Mallouk. 2004. Delivery Vehicles for Zerovalent Metal Nanoparticles in Soil and Groundwater. Chemistry of Materials. 16:2187-2193.
Temple University. 2004. Researchers Using Proteins to Develop Nanoparticles to Aid in Environmental Remediation. ScienceDaily. Available at: http://www.sciencedaily.com/releases/2004/09/040901090324.htm. Accessed February 13, 2008.
Theron, J., J.A. Walker, and T.E. Cloete. 2008. Nanotechnology and Water Treatment: Applications and Emerging Opportunities. Critical Reviews in Microbiology. 34(1):43-69.
Tratnyek, P.G., M.M. Scherer, T.L. Johnson, and L.J. Matheson, in M. Tarr (Ed.). 2003. Chemical Degradation Methods for Wastes and Pollutants, Environmental and Industrial Applications. CRC, 2003. Print. Available at: http://www.ebs.ogi.edu/tratnyek/temp/TratnyekChptr03.pdf![]() .
.
Tratnyek, P.G., and R.L. Johnson. 2006. Nanotechnologies for Environmental Cleanup. Nanotoday. 1(2). Available at: http://dx.doi.org/10.1016/S1748-0132(06)70048-2. Accessed May 20, 2009.
Tungittiplakorn, W., C. Cohen, and L.W. Lion. 2005. Engineered Polymeric Nanoparticles for the Bioremediation of Hydrophobic Contaminants. Environmental Science & Technology. 39:1354-1358.
U.S. Environmental Protection Agency (U.S. EPA). 1999. An In Situ Permeable Reactive Barrier for the Treatment of Hexavalent Chromium and Trichloroethylene in Groundwater: Volume 1: Design and Installation. EPA 600-R-99-095a. Available at: http://www.epa.gov/nrmrl/pubs/600R99095/prbdesign_v1.pdf![]() .
.
U.S. EPA. 2007A. Nanotechnology: Solutions, Challenges, and Implications for Superfund. National Association of Remedial Project Managers Annual Training Conference. Baltimore, Maryland, May 21-25, 2007.
U.S. EPA. 2007B. Science Policy Council. Nanotechnology White Paper. U.S. Environmental Protection Agency. February 2007. Available at: http://www.epa.gov/ncer/nano/publications/whitepaper12022005.pdf![]() .
.
U.S. EPA. 2008A. Nanotechnology for Site Remediation Fact Sheet. Solid Waste and Emergency Response. EPA 542-F-08-009. Available at: http://www.clu-in.org/download/remed/542-f-08-009.pdf![]() .
.
U.S. EPA. 2008B. Office of Superfund, Remediation and Technology Innovation. Nanotechnology: Practical Considerations for Use in Groundwater Remediation. National Association of Remedial Project Managers Annual Training Conference. Portland, Oregon, July 7-11, 2008.
U.S. EPA. 2009. Nanomaterial Research Strategy, Office of Research and Development. EPA 620-K-09-011. Available at: http://www.epa.gov/nanoscience/files/nanotech_research_strategy_final.pdf![]() .
.
Varma R. 2008. Greener Synthesis of Noble Metal Nanostructures and Nanocomposites. Presented at the U.S. EPA Science Forum: Innovative Technologies - Key to Environmental and Economic Progress. May 20 - 22, 2008.
Wang, W., M. Zhou, Z. Jin, and T. Li. 2010. Reactivity Characteristics of Poly(methyl methacrylate) Coated Nanoscale Iron Particles for Trichloroethylene Remediation. Journal of Hazardous Materials, 173: 724-730.
Wilson, G. 2004. Demonstration of In Situ Dehalogenation of DNAPL Through Injection of Emulsified Zero-Valent Iron at Launch Complex 34 in Cape Canaveral Air Force Station, FL. Presented at the Battelle Conference on Nanotechnology Applications for Remediation: Cost-Effective and Rapid Technologies Removal of Contaminants From Soil, Groundwater and Aqueous Environments. September 10, 2004.
Xu J-C., L. Mei, X-Y. Guo, and H-U. Li. 2005. Zinc Ions Surface-Doped Titanium Dioxide Nanotubes and Its Photocatalysis Activity for Degradation of Methyl Orange in Water. Journal of Molecular Catalysis A: Chemical. 226(1):123-127.
Xu Y. and D. Zhao. 2006. Removal of Lead From Contaminated Soils Using Poly(amidoamine) Dendrimers. Industrial & Engineering Chemistry Research. 45:1758-1765.
Zhang, W-X., and D.W. Elliot. 2006. Applications of iron nanoparticles for groundwater remediation. Remediation. 16(2).
Zhang, W-X. 2003. Nanoscale Iron Particles for Environmental Remediation: An Overview. Journal of Nanoparticle Research. 5:323-332.
Zhang, W-X. 2009. Soy Protein and/or Soy Derivatives With Zero-Valent Iron Compositions and Use For Environmental Remediation. U.S. Patent # US 7,507,345 B2.





