Martinsburg Shale - dark gray to black -carbonaceous and fissile - interbedded with thin layers of siltstone and sandstone. Water table in bedrock approximately 30' bgs.
Targeted Environmental Media:
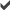 Dense Non-aqueous Phase Liquids (DNAPLs) Dense Non-aqueous Phase Liquids (DNAPLs) 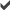 Fractured Bedrock Fractured Bedrock
Major Contaminants and Maximum Concentrations:
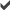 1,1-Dichloroethane (89,000 µg/L) 1,1-Dichloroethane (89,000 µg/L)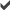 1,1,1-Trichloroethane (1,000,000 µg/L) 1,1,1-Trichloroethane (1,000,000 µg/L) Tetrachloroethene (20 µg/L) Tetrachloroethene (20 µg/L) Trichloroethene (20,000 µg/L) Trichloroethene (20,000 µg/L)
 Borehole Geophysics Borehole Geophysics
- Natural Gamma
- Caliper
- Acoustic Televiewer
- Other
 Fluid Loggings Fluid Loggings
- Temperature
- Conductivity/Resistivity
 Vertical Chemical Profiling Vertical Chemical Profiling  Other Other
Comments:
acoustic velocity, variable density, vertical hydraulic gradient, horizontal gradient.
 Chemical Oxidation (In Situ) Chemical Oxidation (In Situ)
Comments:
This was a pilot scale test. Geophysical logs and packer tests in boreholes were used to determine injector locations and contaminant characteristics of water bearing fractures.
To determine the level of contaminant distruction in bedrock.
During the fourth post injection sampling round (1 year after the pilot study) the reductions in total VOCs ranged from 27% to 86%. Additional injectors were installed to locate the upgradient and downgradient edges of the free phase product
Destruction of the contaminants was qualified by chloride measurements. Vigorous reaction caused the release of oxidation fluids, sediments, etc., to the ground surface in pools. Some post-injection sampling indicated increases in VOC concentrations. This could be due to turbulence from the reaction which mobilized more organics than could be destroyed by the radicals andor because the reaction increased the temperatures which may have increased the solubility of the organic contaminants.
|

