
Ground Water Currents, September 1998, Issue No. 29
Contents
Natural Attenuation of Chlorinated VOCs in Wetlands
Enhancing Aquifer Reclamation through Selective Colloid Mobilization
Vadose Partitioning Interwell Tracer Test for DNAPL Investigation
EPA Solicitation for Small Business Innovation Research
Natural Attenuation of Chlorinated VOCs in Wetlands
by Lisa D. Olsen and Michelle M. Lorah, U.S. Geological Survey, Baltimore, Maryland
The U.S. Geological Survey (USGS) investigated the natural attenuation of chlorinated volatile organic compounds (VOCs) at Aberdeen Proving Ground, MD. Investigations focused on a contaminant plume that discharges from a sand aquifer, through organic-rich wetland sediments, to a freshwater tidal creek. Over a five-year period, the hydrogeology and geochemistry along wetland flow paths and the rates of biodegradation and sorption were studied. Several major findings of the study indicate that natural attenuation is occurring at the West Branch Canal Creek site. Based on the results of this study, natural attenuation has been proposed as a clean-up remedy in an interim record of decision for the West Branch Canal Creek site.
Between World War I and the late 1970s, the area along the West Branch Canal Creek was used to develop, test, and manufacture military-related chemicals. Wastes from these processes were discharged into the creek and surrounding marsh. As a result, contaminants migrated to the underlying aquifer. Compounds identified in the plume include trichloroethylene (TCE), 1,1,2,2-tetrachloroethane (PCA), carbon tetrachloride, and chloroform. The relatively thin layers of wetland sediments were shown to reduce contaminant concentrations in the ground water before it discharges to the wetland surface and adjacent tidal creek.
In the aquifer, concentrations of the parent compounds TCE and PCA ranged from about 100 to 2,000 parts per billion, whereas concentrations of daughter products were low or undetectable. In contrast, parent compounds commonly were not detected in the wetland sediment porewater, but the daughter compounds 1,2-dichloroethylene (1,2-DCE), vinyl chloride (VC), 1,1,2-trichloroethane, and 1,2-dichloroethane were observed. Thus, the presence of daughter products in the naturally anaerobic wetland sediments indicate that TCE and PCA have been degraded by reductive dechlorination reactions. A conceptual model of the natural attenuation processes occurring in this area is shown in Figure 1.
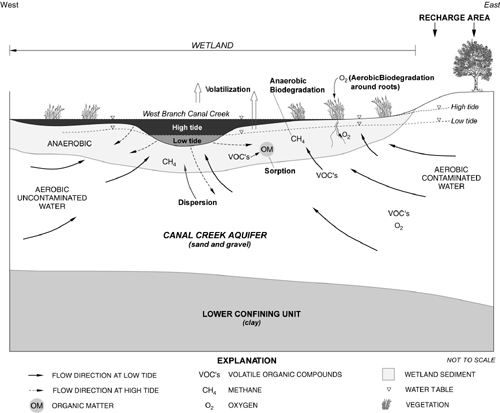
Figure1. Conceptual Model for the West Branch Wetland
Site
Biodegradation processes were evaluated through field evidence of the occurrence of parent and daughter compounds. In addition, the distribution of redox-sensitive constituents, such as methane, sulfate and sulfide, ferric and ferrous iron, and dissolved oxygen, were studied. Biodegradation processes were studied further in anaerobic and aerobic microcosms.
In anaerobic microcosms, maximum potential first-order rate constants for biodegradation of TCE and PCA in the wetland sediments ranged from 0.10 to 0.31 per day under methanogenic conditions, corresponding to half-lives of 2-7 days. The rate constant for TCE biodegradation under sulfate-reducing conditions was 0.045 per day (half-life of 15 days). These estimated rate constants are 2-3 orders of magnitude higher than those reported in the literature for TCE biodegradation in microcosms constructed from sandy aquifer sediments. Reported differences may be attributed to the high percentage (18%) of organic material in the wetland sediments.
In aerobic microcosms, biodegradation rates for cis-1,2-DCE, trans-1,2-DCE, and VC were in the same range as those measured for TCE and PCA under anaerobic conditions. This finding indicates that the production of these daughter products by anaerobic biodegradation of TCE and PCA could be balanced by their consumption where oxygen is available in the wetland sediment, such as near roots and land surface. Researchers also found that, under aerobic conditions, biodegradation of cis-1,2-DCE, trans-1,2-DCE, and VC occurred only in the presence of methane consumption. This indicates that methanotrophs are involved in the biodegradation process. Aerobic biodegradation was fastest for VC and slowest for TCE, showing that faster degradation by methane-utilizing cultures occurs when the compounds are less halogenated.
Analysis of equilibrium sorption isotherms indicated that advective water flow would cause the movement of contaminants in the wetland sediments to be 6-10 times faster than contaminant sorption alone. Currently, the USGS is conducting small-scale tracer tests to better quantify ground-water flow rates, dispersion, and volatilization in the wetland sediments.
Detailed information on this study is provided in the report Natural Attenuation of Chlorinated Volatile Organic Compounds in a Freshwater Tidal Wetland, Aberdeen Proving Ground, Maryland (USGS Water-Resources Investigations Report 97-4171), which may be obtained from the USGS at 303-202-4700. The USGS is continuing to monitor natural attenuation rates at the West Branch Canal Creek site. For additional information, contact Michelle Lorah (USGS) at 410-238-4301 or e-mail mmlorah@usgs.gov.
Enhancing Aquifer Reclamation through Selective Colloid Mobilization
by John C. Seaman, Ph.D., and Paul M. Bertsch, Ph.D., University of Georgia
Research at the Savannah River Ecology Laboratory (SREL) of the University of Georgia has resulted in the development of an in situ means for controlling the mobilization and subsequent transport of native colloidal iron oxides to enhance the efficiency of pump-and-treat remediation. This technology, known as Selective Colloid MobilizationTM (SCM), was developed in partnership with the U.S. Department of Energy (DOE) in an effort to reduce the costs, shorten project durations, and increase the effectiveness of conventional ground-water remediation methods.
The SCM process employs non-hazardous chemical compounds such as calcium or cationic surfactants to mobilize iron oxide colloids, which commonly serve as a primary resident phase for many contaminants in highly weathered, low-carbon systems. Ground water containing the mobilized colloids and associated contaminants is pumped from a contaminated aquifer to ground surface, where the pH of the ground water is adjusted to promote coagulation of the colloids. Following removal of the coagulated colloids, the ground water is returned to the aquifer and the SCM process is repeated until clean-up goals are met.
In a series of intermediate-scale column experiments, coarse-textured, oxide-coated Atlantic Coastal Plain sediments were leached with solutions containing calcium salts or cationic surfactants at various ionic strengths and pH levels. Colloid dispersion resulted from mild changes in solution chemistry. The introduction of dilute calcium chloride solutions resulted in colloid mobilization and in a decrease in effluent pH attributed to aluminum cation exchange and hydrolysis reactions, as well as specific cation sorption reactions on hydrous oxide surfaces that can impart greater net-positive charge. The use of cationic surfactants was even more effective at mobilizing iron oxides without inducing column plugging, a major cause of formation damage that often plagues pump and treat reclamation systems.
In collaboration with Westinghouse-Savannah River, SREL researchers recently completed an extensive series of underground injection experiments that evaluated the potential for formation damage adjacent to injection wells resulting from the long-term application of reclaimed ground water. Although these studies did not evaluate the patented SCM technology directly, they confirmed the basic geochemical processes on which the technology was based.
In these injection well experiments, approximately 40,000 gallons of treated water from a pilot-scale treatment plant were used. Ground-water samples were collected and either filtered or digested using EPA Method #3015 to discriminate between soluble contaminant metals and metals associated with suspended colloids, respectively. Sample turbidity was determined to evaluate the potential for colloid mobilization, and a correlation coefficiency for sample turbidity compared to sample metal concentration was estimated. Higher correlations, and accordingly higher metal concentrations, were identified in most of the digested samples (containing metals associated with suspended colloids) than in the filtered samples (containing metals in a soluble state) (Figure 2). These results confirmed that many of the metals of regulatory importance were associated with colloids (primarily iron oxides) that could be mobilized in response to changes in solution chemistry.
| Figure 2. Correlation Coefficients for Sample Turbidity (NTU) Compared to Metal Concentrations in Filtered and Digested Ground-Water Samples. | ||
| Variable | Filtered | Digested |
|---|---|---|
| NTU | 1.000 | 1.000 |
| Al | 0.147 | 0.247 |
| Ba | -0.018 | 0.880 |
| Ca | -0.025 | 0.315 |
| Cd | -0.076 | -0.060 |
| Cs | 0.239 | 0.803 |
| Fe | 0.115 | 0.942 |
| K | -0.243 | 0.957 |
| Na | 0.240 | 0.183 |
| Pb | 0.028 | 0.964 |
| Rb | 0.213 | 0.977 |
| Sr | -0.067 | 0.855 |
| U | 0.161 | 0.904 |
SCM application is limited to highly weathered, oxide-coated sediments similar to those found on the Atlantic Coastal Plain (a geographic region extending from Mississippi to New Jersey) and regions of Asia and the Far East. The process is effective in the removal of most radionuclides, metals, hydrophobic organics, and oxyanions such as arsenate.
During 1998-1999, SCM field application will be conducted at a selected DOE site. Contact Dr. Paul Bertsch (University of Georgia) at 803-725-5637 or e-mail bertsch@srel.edu, or Dr. John Seaman (University of Georgia) at 803-725-0977 or e-mail seaman@srel.edu for more information.
Vadose Partitioning Interwell Tracer Test for DNAPL Investigation
by Gary A. Pope, Ph.D., University of Texas, and Paul Mariner, Duke Engineering & Services
The University of Texas at Austin, TX, conducted possibly the first field gaseous partitioning interwell tracer test (PITT) to measure contamination from dense non-aqueous phase liquid (DNAPL) in the vadose zone. This testing, which was conducted in December 1995 in cooperation with Duke Engineering & Services, provided important data for future DNAPL remediation.
The PITT was applied in unsaturated alluvium beneath two side-by-side organics disposal trenches at the Chemical Waste Landfill, Sandia National Laboratories, Albuquerque, NM. The water table at this site is approximately 500 feet below the landfill. Residual DNAPL consisted of a mixture of trichloroethene (TCE) and other chlorinated solvents, high-molecular weight hydrocarbons, and polychlorinated biphenyl (PCB) oils.
One injection well and one extraction well were installed 55 feet apart on opposite sides of the target area. Each well was screened at intervals of 10-35 feet, 40-60 feet, and 65-80 feet below ground surface. After steady-state air injection and extraction rates were established, a slug of non-partitioning tracers (methane and sulfur hexafluoride [SF6]) and TCE DNAPL-partitioning gaseous tracers was added to the injection stream. Octafluorocyclo-butane, dodecafluorodimethylcyclobutane, perfluoro-1,3-dimethylcyclohexane, and perfluoro-1,3,5-trimethylcyclohexane (C9F18) served as the TCE DNAPL-partitioning tracers. The respective TCE partition coefficients (mg/L in TCE per mg/L air) for these partitioning tracers were measured in the laboratory to be 9, 16, 72, and 162.
In addition, a water-partitioning tracer, difluoromethane (with a water-air partition coefficient of approximately 1.7 and a TCE-air partition coefficient of approximately 2.0), was included to measure water saturations in the targeted zones. Though the water-partitioning tracer also partitions into TCE, the effect of TCE on overall retardation is negligible when TCE saturation is less than 5 percent of water saturation, as it was in this case. Tracer concentration breakthrough curves at the extraction well and monitor locations were obtained using on-line chromatographs.
SF6 and C9F18 provided the most reliable non-partitioning and TCE DNAPL-partitioning tracer data for this test. Initial analysis indicated that TCE DNAPL penetrated non-uniformly to a depth of 20-30 feet below ground surface. Multi-level monitor well data showed that TCE decreased in volume with increasing depth. The average TCE saturation in the shallow zone between 10 and 35 feet below ground surface was measured to be 0.11 percent, based on a calculated C9F18 retardation factor of 1.2. One monitor point at 10 feet below ground surface indicated a TCE saturation of 0.25 percent, corresponding to a retardation factor of approximately 1.4 (Figure 3). No TCE DNAPL was detected in the intermediate or deep zones. Average water saturations in the shallow, intermediate, and deep zones were measured to be 23 percent, 13 percent, and 10 percent, respectively.
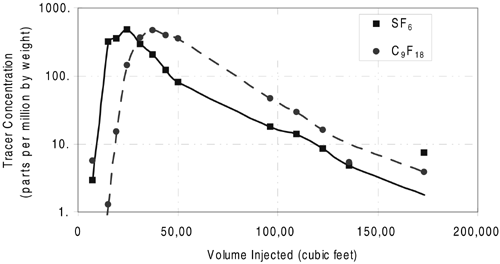
Figure 3. SF6 and C9F18 Tracer
Response at Shallow Monitor Point.
The PITT results provided a basis for deciding the voluntary corrective action strategy at the site. In addition to allaying fears that DNAPL had penetrated to the water table, the results show that a combination of excavation and vapor extraction would satisfy clean-up objectives. For more information on the PITT (patent pending), contact Dr. Gary Pope (University of Texas) at 512-471-3235 or e-mail gary_pope@brazos.pe.utexas.edu, or Paul Mariner (Duke Engineering & Services) at 970-256-0535 or e-mail pemarine@dukeengineering.com.
EPA Solicitation for Small Business Innovation Research
A solicitation for research proposals from science and technology-based firms will open on September 17, 1998, and close on November 19, 1998. “Phase I” contracts of up to $70,000 will be awarded to small businesses for investigation of the scientific merit and technical feasibility of proposed technologies or products. Recipients of Phase I contracts will be eligible to compete for “Phase II” contracts of up to $295,000 to complete the research and development required for technology or product commercialization.
The solicitation will be posted on the National Center for Environmental Research and Quality Assurance Web site at: http://www.epa.gov/ncerqa; users may click on “Small Business” to access the current (1999) solicitation and “Archive” to view last year’s solicitation. For additional assistance, call the EPA Small Business Innovation Research Helpline at 1-800-490-9194.
| We now offer a new service-TechDirect-to keep you abreast of new EPA publications and event of interest to site remediation and site characterization professionals. Once a month, a TechDirect message will be sent via email describing new products and instructions on how to obtain them. |







