AOP Treats Dioxane-Contaminated Ground Water
The San Gabriel Basin Water Quality Authority is working with private industry to examine options for removing 1,4-dioxane in ground water undergoing chlorinated solvent removal at two former industrial sites in Southern California. A pump and treatment system employing liquid-phase granular activated carbon (LGAC) has operated at one of the sites, in South El Monte, since 1995 to remove chlorinated solvents. The system was found ineffective at removing 1,4-dioxane, which was discovered in 1999 at concentrations reaching 20 µg/L. A pilot-scale advanced oxidation process (AOP) using ex-situ injection of ozone and hydrogen peroxide was implemented for one month in 2001 to address the 1,4-dioxane.
The AOP is a continuous, in-line process operating at feed water pressure with a flow of 10-1,000 gpm. The 10-gpm AOP mobile unit used during the pilot contains a solid-state ozone generator producing ozone at a pressure of 40-50 psig. The unit also houses a hydrogen peroxide delivery system that uses a continuous reactor to inject hydrogen peroxide initially at a single point, followed by ozone at multiple points along the flow path. Earlier testing showed that multiple small-scale injections, rather than a single large-scale event, increased the process effectiveness and minimized byproduct formation.
The continuous reactor initially contained 18 individual reactors, each with three separate sections for injection, mixing, and chemical reaction (Figure 1), and sampling ports at the point of effluent. Depending on the rate of flow, residence time within each of the individual reactors was 3-10 seconds. Ozone and hydrogen peroxide initially were applied at rates of 9.4 ppm and 14.2 ppm, respectively. Approximately 0.7 moles of peroxide solution were injected into the influent for each mole of ozone applied within the reactor. Subsequent system optimization reduced the ozone and hydrogen peroxide application rates to 3.1 ppm and 6.9 ppm, respectively, and reduced the number of required reactors to 3.
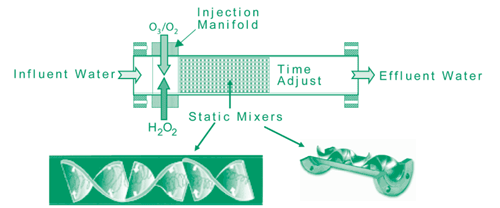
Treatment resulted in a decrease of the 1,4-dioxane concentration to a level below the State of California drinking water standard (3 µg/L) and a 98% decrease in most other chlorinated solvents. Based on the pilot’s success, a full-scale AOP was constructed in 2002 and has operated for the past year as a continuous pre-treatment step. The system comprises three ozone injectors with eight static mixers operating at a capacity of 500 gpm.
Early monitoring results of the full-scale operation demonstrate the same removal rates for 1,4-dioxane and chlorinated solvents as those observed during the pilot project. Data indicate that addition of the AOP system is increasing the life of the LGAC, which is now replaced semi-annually rather than quarterly. As a result of 1,4-dioxane removal, effluent from the LGAC system now can be reinjected into ground water approximately 1-2 miles upgradient of a nearby drinking water well field to serve as a barrier.
Similar success was demonstrated in a pilot AOP system operated in the City of Industry. At this location, 1,4-dioxane was detected in extraction wells that feed an air stripper used to remove chlorinated solvents. Concentrations of 1,4-dioxane were found to decrease consistently from 610 to 4 ppb during AOP pre-treatment. Researchers believe that a further reduction in 1,4-dioxane concentrations would have been achieved with higher ozone dosage. A full-scale system with a 70-gpm capacity has been installed at this site for use as a pre-treatment step prior to air stripping.
Due to the recent discovery of 1,4-dioxane in ground water throughout California, the Santa Clara Valley Water District anticipates continued need for 1,4-dioxane monitoring.
Contributed by Reid Bowman, Ph. D., Applied Process Technology, Inc. (805-649-5796 or rbowman@aptwater.com) and Tom Mohr, Santa Clara Valley Water District (408-265-2607 or tmohr@valleywater.org)
ETV Program Verifies Performance of Ground-Water Sampling Devices
The EPA-sponsored Environmental Technology Verification (ETV) program has established several testing centers over the past 10 years to verify new technologies for a variety of environmental applications. One of these centers—the Advanced Monitoring Systems Center (AMS)—recently tested eight ground-water sampling devices: six devices (including bladder pumps, grab samplers, passive sampling devices, and a down-well sampling module), suitable for deployment in conventional, 2-inch diameter and larger wells and two devices deployable in 1-inch or smaller diameter wells installed by direct-push methods.
As one of several third-party, independent testing organizations within AMS, Sandia National Laboratories designed and conducted the tests in cooperation with the technology vendors. Tests were performed under well-controlled conditions in a test facility and under less experimental control at contaminated ground-water sites.
Controlled testing was conducted at a 100-foot standpipe at the U.S. Geological Survey’s Hydrological Instrumentation Facility at the NASA Stennis Space Center, MS. The standpipe is housed in a former Saturn V rocket hangar with multiple access platforms along the length of the standpipe. Large mixing tanks and a water supply at the top of the pipe allow contaminant-spiked solutions to be prepared and dispersed into the standpipe. Ground-water sampling devices were deployed in the pipe from the top in the same manner they would be deployed in an onsite monitoring well. The 5-inch diameter, stainless-steel standpipe was equipped with multiple external access ports along its length to allow collection of co-located reference samples at the same time that vendors collected samples from inside the pipe.
Field testing of the conventional larger-scale devices was conducted in a ground-water monitoring well field at the NASA Stennis site. Ground water in this region is contaminated with a variety of volatile organic compounds (VOCs)—predominately chlorinated solvents—resulting from former machine shop operations and solvent disposal. VOC concentrations in ground water at the test area ranged from single digit parts per billion levels to tens of parts per million.
Field testing for the smaller direct-push well devices was conducted at Tyndall Air Force Base, FL. Several areas of contaminated ground water exist at the site as a result of past disposal of solvents used in aircraft maintenance. With its existing network of 1-inch diameter, direct-push wells, Tyndall AFB is one of several military installations involved in long-term comparison of conventionally installed wells and direct-push wells at contaminated sites. VOC concentrations in ground water at the test area ranged from single digit parts per billion to hundreds of parts per billion.
Performance of the following ground-water sampling devices was verified in cooperation with vendors:
- Multiprobe 100 (Burge Environmental, Inc.)
- SampleEase SP15T36 (Clean Environmental Equipment)
- Micro-Flo 57400 (GeoLog, Inc.)
- Gore-Sorber Water Quality Monitor (W.L. Gore and Associates)
- Kabis Sampler I/II (Sibak Industries)
- Well Wizard Dedicated Sampling System T120M/T1250 (QED Environmental Systems, Inc.)
- Mechanical Bladder Pump MB470 (Geoprobe Systems, Inc.), and
- Pneumatic Bladder Pump GW1400 (Geoprobe Systems, Inc.).
Each device was used to collect 100 or more standpipe and ground-water samples and all vendor samples were matched with reference samples. Performance was determined for sampler precision, comparability with a reference, versatility, and logistical requirements. Typical precision and relative accuracy performance data for standpipe VOCs are summarized in Figures 2 and 3.
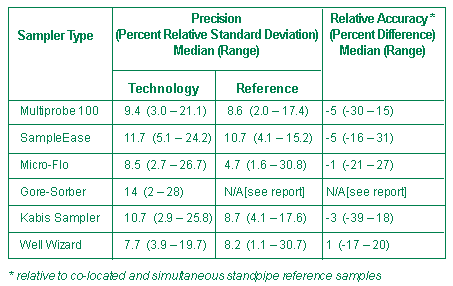
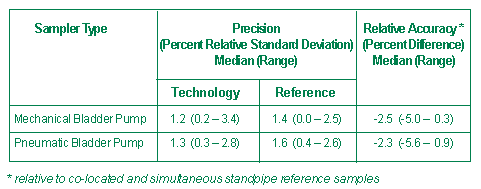
In general, test results revealed that all tested sampling devices are suitable for use in various ground-water sampling applications. The published performance verification data for each device can be used to optimize the choice of a sampler in a particular field application. A complete performance report on each of these ground-water sampling devices is available on the ETV website (http://www.epa.gov/etv).
Contributed by Wayne Einfeld, Sandia National Laboratories (505-845-8314 or weinfel@sandia.gov)
ESTCP Evaluates Bimetallic Nanoscale Particles in Treating CVOCs
Under the Environmental Security Technology Certification Program (ESTCP), the U.S. Department of Defense (DOD) is evaluating the use of bimetallic nanoscale iron (BNI) particles in establishing a permeable, in-situ, reactive zone for treating chlorinated volatile organic compounds (CVOCs) in ground water. When compared to conventional iron-based permeable reactive barriers employing granular particles, nanoscale (less than a micron in size) metallic iron provides more available surface area per unit of iron mass. Preliminary results of the ESTCP study indicate that the increased surface area allows higher levels of reactivity with contaminants, while generating lower iron mass loadings. DOD anticipates that the use of nanoscale particles will enable iron to be injected beneath ground-surface structures, where surface access is otherwise limited by existing land uses, or at depths at which trenching is impractical, with minimal ground surface disruption.
The use of BNI colloids for in-situ remediation builds on the use of nanoscale iron colloids alone, which have been used by others in both bench- and field-scale applications. During this project, analytical results showed that the addition of an incomplete coating (0.03% w/w) of palladium as a hydrogenation catalyst to the surface of nanoscale iron particles increased the CVOC dechlorination rate from a kinetic value of 0.002 L/m2/g to values of 1 L/m2/g or more. Data indicated that the increased surface area provided by nanoscale iron, combined with the increased reaction rate caused by the addition of hydrogenation catalyst, achieved a 100-150% higher treatment rate than those estimated to be achieved by granular iron filings. The addition of palladium also enabled more complete conversion of contaminants into non-toxic gas.
Using BNI produced through two types of colloid manufacturing techniques, laboratory studies were conducted in soil columns packed with soil and treated with ground-water samples taken from a site at Vandenberg Air Force Base, CA, which serves as the primary pilot location for this project. Samples were collected several hundred yards from a missile launch pad drainage channel where trichloroethene (TCE) was found in ground water at a concentration of 2.5 mg/L. The TCE degradation product cis-1,2-dichloroethene (DCE) also was identified, but at lower concentrations.
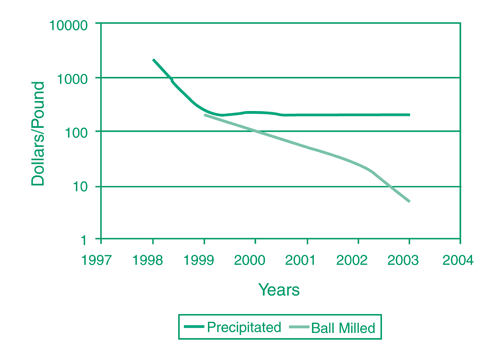
The "aqueous precipitation" technique used to manufacture nanoscale iron particles involves reduction of a solution of ferric chloride using sodium borohydride. In contrast, the "ball milling" technique involves reduction of larger, typically micron-size, iron powder granules to nanoscale size through an attrition process using a suitably sized ball mill. Over three years, this project supported enhancements to the "ball milling" process that have lowered the cost of manufacturing nanoscale iron to $5/lb (Figure 4).
To date, laboratory testing has resulted in the creation of a “library” of kinetic treatment rates indexed by the types of nanoscale iron being tested. The kinetic rate constants normalized for the surface area of iron have ranged from 0.05 L/m2/g to 0.4 L/m2/g. Colloids ranging in size from 200 to thousands of nanometers were tested in the laboratory to determine the optimal size for field application. Results demonstrated that colloids in the range of 200-600 nanometers achieved optimal mobility and a sufficient reactivity rate for in-situ CVOC dechlorination. Colloids of smaller size were subject to adsorptive interfacial forces in the geologic matrix, while larger ones were adversely affected by gravitational settling.
This project has resulted in a cost-effective BNI production process that can be up-scaled to yield nanoscale iron in large quantities (several tons) for full-scale remediation. As it continues, this project will focus on: (1) understanding and manipulating technical factors that improve the reactive life of BNI once it is deployed in-situ; and (2) demonstrating BNI applicability for remediation of CVOCs present as dense, nonaqueous phase liquid (DNAPL) in laboratory soil columns. Updated information on this ongoing ESTCP project is available at http://www.estcp.org.
Contributed by David Liles, ARCADIS (919-544-4535 or dliles@arcadis-us.com) and Andrea Leeson DOD/SERDP (703-696-2118 or Andrea.Leeson@osd.mil)
NRMRL Evaluates Microbial Responses to Ground-Water Remediation Technologies
The U.S. EPA National Risk Management Research Laboratory (NRMRL) recently completed independent evaluation of the microbial responses to technology demonstrations that were conducted over the past several years at Cape Canaveral Air Station, FL. The Interagency DNAPL Consortium had conducted side-by-side field demonstrations of three technologies for treating trichloroethene existing as DNAPL: permanganate-based, in-situ, chemical oxidation (ISCO), six-phase heating (SPH), and steam injection (SI). [Performance summaries are available in the March 2003 Technology News and Trends and July 2001 Ground Water Currents at http://www.clu-in.org]. The recent NRMRL evaluation was conducted in part to address concerns that aggressive source control technologies such as those demonstrated at Cape Canaveral might sterilize the subsurface, thus hampering the use of natural or enhanced bioattenuation processes as remedial finishing steps. Aggressive technologies typically remediate a substantial portion of DNAPL but do not achieve regulatory clean-up levels. Results of the study indicate that sterilization did not occur. Biomass levels following application of all three technologies returned to near pre-treatment levels.
NRMRL's study focused on the analysis of phospholipid ester-linked fatty acids (PLFA) profiles, which provide information on the phylogenic identity and physiological status of microbes. Individual fatty acids are known to differ in chemical composition that depends on microbial type and environmental conditions. These differences allow fatty acids to be used as "biomarkers" providing a quantitative insight into three primary attributes of microbial communities: viable biomass, community structure, and metabolic activity.
During the three-year NRMRL evaluation, PLFA distribution and content were determined from 266 core samples extracted aseptically at depths of 6-44 feet. Samples were collected at a site control with no DNAPL contamination and in the areas where each of the three demonstrations occurred (Figure 5).
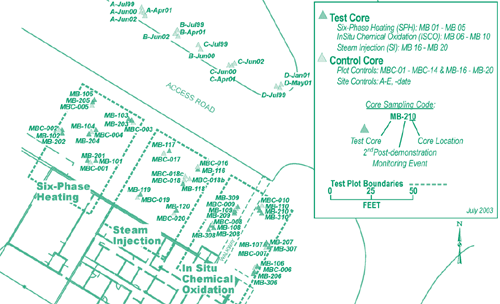
Comprehensive spatial and temporal screening data suggested that the technology applications did not significantly alter the site’s microbial community structure. PLFA distribution at the site control suggested that the microbial community structures were atypical. In particular, the biomass gradient did not decrease with increasing depth. The high level of biomass variation identified at each depth, however, was consistent with the extensive heterogeneity of biota expected in subsurface environments. Analysis of fatty acid structural groups indicated that short saturates constituted the largest group and that the number of long saturates was significantly higher than usual. These findings contrasted with other studies indicating that monosaturated PLFA usually are the most abundant in subsurface conditions, while long saturates constitute a fewer percent of the total fatty acids.
PLFA distribution for each of the three demonstration areas also indicated a high variation in biomass at each sampling event and depth. ISCO was the only technology found to stimulate microbial abundance; a significant initial increase in biomass was observed following completion of the ISCO demonstration. This behavior is consistent with findings from other permanganate-based chemical oxidation applications. Biomass and the proportion of monounsaturates returned to normal levels shortly after chemical injections ceased.
In general, no significant change in the microbial community composition was observed in the SPH or SI treatment areas at Cape Canaveral. Microbial communities recovered to near initial conditions by the second sampling event performed for both the SPH and SI demonstrations.
Limiting factors for this evaluation may include the selection of fatty acid structural groups that may have been insufficiently sensitive to subtle differences in microbial populations, or the use of samples with very low biomass and corresponding patterns of PFLA. Although the PLFA tests suggest that the indigenous microbial population recovered after treatments, further investigations are required to demonstrate conclusively that the population can be used as a finishing step. Details on the general approach used for sampling and analysis during this study will be available in a 2004 environmental research brief available at http://www.epa.gov/ORD/NRMRL/Pubs/index.html.
Contributed by Ann Azadpour-Keeley, NRMRL (580-436-8890 or keeley.ann@epa.gov)





